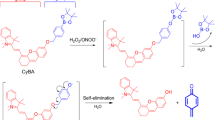
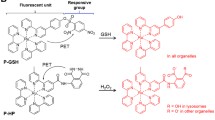
Abstract
Purpose
This study describes an imaging strategy based on glow stick chemistry to non-invasively image oxidative stress and reactive oxygen species (ROS) production in living animals.
Procedures
Upon stimulation, phagocytes produce toxic levels of ROS to kill engulfed microorganisms. The mitochondrial respiratory chain continually generates low levels of superoxide (O2·−) that serve as a source for generation of downstream ROS, which function as regulatory signaling intermediaries. A ROS-reacting substrate, 2-methyl-6-[4-methoxyphenyl]-3,7-dihydroimidazo[1,2-a]pyrazin-3-one hydrochloride, was used as the chemical energy donor for generating energy transfer luminescence in phagosomes and mitochondria.
Results
Using targeted photoluminescent dyes with specific subcellular localization that serve as chemical energy recipients, our imaging data demonstrate proof-of-concept for using glow stick chemistry to visualize ROS production associated with phagocytosis and mitochondrial respiration in living mice.
Conclusions
Glow stick imaging is a complementary hybrid of chemiluminescence and photoluminescence imaging, capable of generating red or far-red emission for deep tissue imaging.





Similar content being viewed by others
References
Leblond F, Davis SC, Valdes PA, Pogue BW (2010) Pre-clinical whole-body fluorescence imaging: review of instruments, methods and applications. J Photochem Photobiol B 98:77–94
Ntziachristos V, Bremer C, Weissleder R (2003) Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol 13:195–208
Roda A, Pasini P, Mirasoli M et al (2004) Biotechnological applications of bioluminescence and chemiluminescence. Trends Biotechnol 22:295–303
Contag CH, Bachmann MH (2002) Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng 4:235–260
Greer LF III, Szalay AA (2002) Imaging of light emission from the expression of luciferases in living cells and organisms: a review. Luminescence 17:43–74
Rauhut MM (1969) Chemiluminescence from concerted peroxide decomposition reactions. Acc Chem Res 2:80–87
Rauhut MM, Bollyky LJ, Roberts BG et al (1967) Chemiluminescence from reactions of electronegatively substituted aryl oxalates with hydrogen peroxide and fluorescent compounds. J Am Chem Soc 89:6515–6522
Pfleger KD, Eidne KA (2006) Illuminating insights into protein–protein interactions using bioluminescence resonance energy transfer (BRET). Nat Methods 3:165–174
Freeman R, Liu X, Willner I (2011) Chemiluminescent and chemiluminescence resonance energy transfer (CRET) detection of DNA, metal ions, and aptamer-substrate complexes using hemin/G-quadruplexes and CdSe/ZnS quantum dots. J Am Chem Soc 133:11597–11604
Nakano M, Sugioka K, Ushijima Y, Goto T (1986) Chemiluminescence probe with Cypridina luciferin analog, 2-methyl-6-phenyl-3,7-dihydroimidazo[1,2-a]pyrazin-3-one, for estimating the ability of human granulocytes to generate O2. Anal Biochem 159:363–369
Akutsu K, Nakajima H, Katoh T et al (1995) Chemiluminescence of Cipridina luciferin analogues. Part 2. Kinetic studies on the reaction of 2-methyl-6-phenylimidazo[1,2-a]pyrazin-3(7H)-one (CLA) with superoxide: hydroperoxyl radical is an actual active species used to initiate the reaction. J Chem Soc Perkin Trans 2:1699–1706
Kambayashi Y, Ogino K (2003) Reestimation of Cypridina luciferin analogs (MCLA) as a chemiluminescence probe to detect active oxygen species—cautionary note for use of MCLA. J Toxicol Sci 28:139–148
Tarpey MM, White CR, Suarez E et al (1999) Chemiluminescent detection of oxidants in vascular tissue: lucigenin but not coelenterazine enhances superoxide formation. Circ Res 84:1203–1211
Mitani M, Yokoyama Y, Ichikawa S et al (1994) Determination of horserdish peroxidase concentration using the chemiluminescence of Cypridina luciferin analogue, 2 methyl-6-(p-methyoxyphenyl)-3,7-dihydroimidazo[1,2-a]pyrazin-3-one. J Biolumin Chemilumin 9:355–361
Takahashi A, Totsune-Nakano H, Nakano M et al (1989) Generation of O2 – and tyrosine cation-mediated chemiluminescence during the fertilization of sea urchin eggs. FEBS Lett 246:117–119
Baker CJ, Deahl K, Domek J, Orlandi EW (2000) Scavenging of H2O2 and production of oxygen by horseradish peroxidase. Arch Biochem Biophys 382:232–237
Babior BM (1999) NADPH oxidase: an update. Blood 93(5):1464–1476
Gross S, Gammon ST, Moss BL et al (2009) Bioluminescence imaging of myeloperoxidase activity in vivo. Nat Med 15:455–461
Tseng JC, Kung AL (2012) In vivo imaging of inflammatory phagocytes. Chem Biol 19:1199–1209
Green K, Brand MD, Murphy MP (2004) Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53(Suppl 1):S110–S118
Smiley ST, Reers M, Mottola-Hartshorn C et al (1991) Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A 88:3671–3675
Ogata E, Rasmussen H (1966) Valinomycin and mitochondrial ion transport. Biochemistry 5:57–66
Brand MD (2010) The sites and topology of mitochondrial superoxide production. Exp Gerontol 45:466–472
Tseng JC, Wang Y, Banerjee P, Kung AL (2012) Incongruity of imaging using fluorescent 2-DG conjugates compared to 18F-FDG in preclinical cancer models. Mol Imaging Biol 14:553–560
Kessler RJ, Tyson CA, Green DE (1976) Mechanism of uncoupling in mitochondria: uncouplers as ionophores for cycling cations and protons. Proc Natl Acad Sci U S A 73:3141–3145
Freeman BA, Crapo JD (1981) Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem 256:10986–10992
Churchill EN, Szweda LI (2005) Translocation of deltaPKC to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Arch Biochem Biophys 439:194–199
Hsu PP, Sabatini DM (2008) Cancer cell metabolism: Warburg and beyond. Cell 134:703–707
Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7:97–110
Chen LB (1988) Mitochondrial membrane potential in living cells. Annu Rev Cell Biol 4:155–181
Collins TJ, Berridge MJ, Lipp P, Bootman MD (2002) Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J 21:1616–1627
Osman AM, Laane C, Hilhorst R (2001) Enhanced sensitivity of Cypridina luciferin analogue (CLA) chemiluminescence for the detection of *O2(−) with non-ionic detergents. Luminescence 16:45–50
Lee D, Khaja S, Velasquez-Castano JC et al (2007) In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat Mat 6:765–769
Dragulescu-Andrasi A, Chan CT, De A et al (2011) Bioluminescence resonance energy transfer (BRET) imaging of protein–protein interactions within deep tissues of living subjects. Proc Natl Acad Sci U S A 108:12060–12065
So MK, Xu C, Loening AM, Gambhir SS et al (2005) Self-illuminating quantum dot conjugates for in vivo imaging. Nat Biotechnol 24:339–343
Xiong L, Shuhendler AJ, Rao J (2012) Self-luminating BRET-FRET near-infrared dots for in vivo lymph-node mapping and tumour imaging. Nat Commun 3:1193
Zhang N, Francis KP, Prakash A, Ansaldi D (2013) Enhance detection of myeloperoxidase activity in deep tissues through luminescent excitation of near-infrared nanoparticles. Nat Med 19:500–505
Acknowledgments
This work was supported by the Nancy Lurie Marks Foundation. We thank Kin K. Wong for his assistance in preparing this report. We thank Dr. Lisa Cameron at DFCI for providing technical support of microscopic imaging. We thank Dr. Nancy E. Kohl and Dr. Annick Van den Abbeele for critical reading and insightful discussion.
Conflict of Interest
D.B., T.T., and A.L.K. declare no conflict of interest in this study. J.–C.T. submitted a patent application based on this study (DFCI).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 6.73 MB)
Rights and permissions
About this article
Cite this article
Tseng, JC., Bailey, D., Tupper, T. et al. Using Glow Stick Chemistry for Biological Imaging. Mol Imaging Biol 16, 478–487 (2014). https://doi.org/10.1007/s11307-014-0721-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0721-8