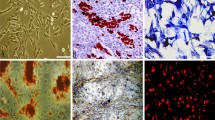
Abstract
Purpose
Transplantation of a regenerative cell population derived from human subcutaneous adipose tissue (hASCs) for cardiac regeneration represents a promising therapy due to the capacity of these cells for proliferation and differentiation. Understanding the fate of injected hASCs would help to understand how hASCs work in vivo. The aim of this study was to track the long-term fate, including survival, differentiation, proliferation, apoptosis, migration, and growth factor secretion of intramyocardially injected hASCs following experimental acute myocardial infarction in an immunodeficient mouse model.
Methods
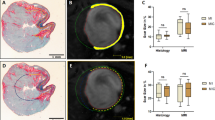
Myocardial infarction was experimentally induced in severe combined immunodeficient mice by permanent ligation of the left anterior descending coronary artery. Lentivirally labeled hASCs (5 × 105; expressing green fluorescence protein [GFP] and luciferase) were injected into the peri-infarct region. Colony formation, growth kinetics, and differentiation of transduced hASCs were analyzed in vitro and compared to those of untransduced hASCs. The survival and migration of injected hASCs were tracked by luciferase-based bioluminescence imaging for 10 weeks. Immunofluorescence and terminal deoxynucleotidyl transferase dUTP nick end labeling staining were used to assess differentiation, proliferation, growth factor expression, or apoptosis of grafted hASCs in infarcted hearts and potential distribution to other tissues.
Results
Lentivirus transduction and GFP and luciferase expression did not influence proliferation or differentiation of hASCs. Bioluminescence imaging demonstrated that injected hASCs survived in infarcted hearts during the follow-up of 10 weeks. Immunofluorescence confirmed that hASCs engrafted in ischemic hearts expressed bFGF and IGF-1, and did not migrate into other organs. Of all engrafted hASCs, 3.5% differentiated into cardiomyocytes or endothelial cells. Other cells maintained their proliferative potential or underwent apoptosis.
Conclusion
Luciferase-based bioluminescence imaging allows long-term tracking of intramyocardially injected hASCs in living mice. The hASCs might enhance function of injured hearts through long-term engraftment, growth factor secretion, and transdifferentiation to cardiomyocytes and endothelial cells.







Similar content being viewed by others
References
Bai X, Ma J, Pan Z et al (2007) Electrophysiological properties of human adipose tissue-derived stem cells. Am J Physiol Cell Physiol 293:C1539–C1550
Zuk PA, Zhu M, Ashjian P et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Bai X, Yan Y, Song YH et al (2010) Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J 31(4):489–501
Alt E, Pinkernell K, Scharlau M, Coleman M, Fotuhi P, Nabzdyk C, Matthias N, Gehmert S, Song YH (2009) Effect of freshly isolated autologous tissue resident stromal cells on cardiac function and perfusion following acute myocardial infarction. Int J Cardiol. doi:10.1016/j.ijcard.2009.03.124
Valina C, Pinkernell K, Song YH et al (2007) Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J 28:2667–2677
Wang L, Deng J, Tian W et al (2009) Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: an MR imaging study of rat hearts. Am J Physiol Heart Circ Physiol 297:H1020–H1031
Leobon B, Roncalli J, Joffre C et al (2009) Adipose-derived cardiomyogenic cells: in vitro expansion and functional improvement in a mouse model of myocardial infarction. Cardiovasc Res 83:757–767
Kummer C, Winkeler A, Dittmar C et al (2007) Multitracer positron emission tomographic imaging of exogenous gene expression mediated by a universal herpes simplex virus 1 amplicon vector. Mol Imaging 6:181–192
Bai X, Pinkernell K, Song YH et al (2007) Genetically selected stem cells from human adipose tissue express cardiac markers. Biochem Biophys Res Commun 353:665–671
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Lee HS, Huang GT, Chiang H et al (2003) Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells 21:190–199
Bai X, Xiao Z, Pan Y et al (2004) Cartilage-derived morphogenetic protein-1 promotes the differentiation of mesenchymal stem cells into chondrocytes. Biochem Biophys Res Commun 325:453–460
Hiba B, Richard N, Thibault H et al (2007) Cardiac and respiratory self-gated cine MRI in the mouse: comparison between radial and rectilinear techniques at 7T. Magn Reson Med 58:745–753
Dellavalle A, Sampaolesi M, Tonlorenzi R et al (2007) Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9:255–267
Chapon C, Jackson JS, Aboagye EO et al (2009) An in vivo multimodal imaging study using MRI and PET of stem cell transplantation after myocardial infarction in rats. Mol Imaging Biol 11:31–38
Kim YJ, Huh YM, Choe KO et al (2009) In vivo magnetic resonance imaging of injected mesenchymal stem cells in rat myocardial infarction; simultaneous cell tracking and left ventricular function measurement. Int J Cardiovasc Imaging 25(Suppl 1):99–109
Hou D, Youssef EA, Brinton TJ et al (2005) Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation 112:I150–I156
Sheikh AY, Lin SA, Cao F et al (2007) Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells 25:2677–2684
Vilalta M, Degano IR, Bago J et al (2008) Biodistribution, long-term survival, and safety of human adipose tissue-derived mesenchymal stem cells transplanted in nude mice by high sensitivity non-invasive bioluminescence imaging. Stem Cells Dev 17:993–1003
Tillmanns J, Rota M, Hosoda T et al (2008) Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci USA 105:1668–1673
Mangi AA, Noiseux N, Kong D et al (2003) Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 9:1195–1201
Wang M, Tan J, Wang Y et al (2009) IL-18 binding protein-expressing mesenchymal stem cells improve myocardial protection after ischemia or infarction. Proc Natl Acad Sci USA 106:17499–17504
Wang X, Zhao T, Huang W et al (2009) Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells 27:3021–3031
Wisel S, Khan M, Kuppusamy ML et al (2009) Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Ther 329:543–550
Sadat S, Gehmert S, Song YH et al (2007) The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun 363:674–679
Peroni D, Scambi I, Pasini A et al (2008) Stem molecular signature of adipose-derived stromal cells. Exp Cell Res 314:603–615
Battler A, Scheinowitz M, Bor A et al (1993) Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infarcted swine myocardium. J Am Coll Cardiol 22:2001–2006
Lee KY, Peters MC, Mooney DJ (2003) Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J Control Release 87:49–56
Sources of Funding
This work was supported by the Alliance of Cardiovascular Researchers grant 543102 (to E. Alt).
Conflicts of Interest
Michael Coleman and Grace Wu are connected with InGeneron, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Significance:
Transplantation of a regenerative cell populations derived from human subcutaneous adipose tissue (hASCs) for cardiac regeneration represents a promising therapy due to the capacity of these cells for proliferation and differentiation. In this study, we systematically assessed the persistence, differentiation, and paracrine fate of intramyocardially injected hASCs for 10 weeks after myocardial infarction. Our results show that (1) injected hASCs survive long-term in infarcted heart, (2) injected hASCs not only secrete growth factors (basic fibroblast growth factor and insulin-like growth factor-1) but also differentiated into cardiomyocytes and endothelial cells, and (3) bioluminescent imaging of genetically labeled hASCs was an effective tool for serial quantitation of cell engraftment and distribution. Understanding the fate of injected hASCs should help to understand how hASCs work in vivo and optimize hASC-based therapeutic strategy for myocardial regeneration.
Rights and permissions
About this article
Cite this article
Bai, X., Yan, Y., Coleman, M. et al. Tracking Long-Term Survival of Intramyocardially Delivered Human Adipose Tissue-Derived Stem Cells Using Bioluminescence Imaging. Mol Imaging Biol 13, 633–645 (2011). https://doi.org/10.1007/s11307-010-0392-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0392-z