Abstract
Purpose
This study was designed to assess changes in brain glucose metabolism in rats after visual stimulation.
Materials and methods
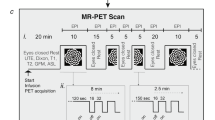
We sought to determine whether visual activation in the rat brain could be detected using a small-animal positron emission tomography (PET) scanner and 2-deoxy-2-[18F]fluoro-d-glucose (FDG). Eleven rats were divided into two groups: (a) five animals exposed to ambient light and (b) six animals stimulated by stroboscopic light (10 Hz) with one eye covered. Rats were injected with FDG and, after 45 min of visual stimulation, were sacrificed and scanned for 90 min in a dedicated PET tomograph. Images were reconstructed by a three-dimensional ordered subset expectation maximization algorithm (1.8 mm full width at half maximum). A region-of-interest (ROI) analysis was performed on 14 brain structures drawn on coronal sections. Statistical parametric mapping (SPM) adapted for small animals was also carried out. Additionally, the brains of three rats were sliced into 20-μm sections for autoradiography.
Results
Analysis of ROI data revealed significant differences between groups in the right superior colliculus, right thalamus, and brainstem (p ≤ 0.05). SPM detected the same areas as the ROI approach. Autoradiographs confirmed the existence of hyperactivation in the left superior colliculus and auditory cortex.
Conclusions
To our knowledge, this is the first report that uses FDG-PET and SPM analysis to show changes in rat brain glucose metabolism after a visual stimulus.



Similar content being viewed by others
References
Lund RD, Lund JS (1965) The visual system of the mole Talpa europaea. Exp Neurol 13(3):302–316
Lund RD, Lund JS, Wise RP (1974) The organization of the retinal projection to the dorsal lateral geniculate nucleus in pigmented and albino rats. J Comp Neurol 158(4):383–403
Guillery RW (1971) An abnormal retinogeniculate projection in the albino ferret (Mustela furo). Brain Res 33(2):482–485
Guillery RW (1974) Visual pathways in albinos. Sci Am 230(5):44–54
Guillery RW (1996) Why do albinos and other hypopigmented mutants lack normal binocular vision, and what else is abnormal in their central visual pathways. Eye 10(Pt 2):217–221
Klooster J, van der Want JJ, Vrensen G (1983) Retinopretectal projections in albino and pigmented rabbits: an autoradiographic study. Brain Res 288(1–2):1–12
Balkema GW (1988) Elevated dark-adapted thresholds in albino rodents. Invest Ophthalmol Vis Sci 29(4):544–549
Balkema GW, Drager UC (1991) Impaired visual thresholds in hypopigmented animals. Vis Neurosci 6(6):577–585
Balkema GW, MacDonald S (1998) Increased absolute light sensitivity in Himalayan mice with cold-induced ocular pigmentation. Vis Neurosci 15(5):841–849
Herreros de Tejada P, Green DG, Munoz Tedo C (1992) Visual thresholds in albino and pigmented rats. Vis Neurosci 9(3–4):409–414
Munoz Tedo C, Herreros de Tejada P, Green DG (1994) Behavioral estimates of absolute threshold in rat. Vis Neurosci 11(6):1077–1082
Naarendorp F, Sato Y, Cajdric A et al (2001) Absolute and relative sensitivity of the scotopic system of rat: electroretinography and behavior. Vis Neurosci 18(4):641–656
Fitzpatrick DC, Batra R, Stanford TR et al (1997) A neuronal population code for sound localization. Nature 388(6645):871–874
King AJ, Bajo VM, Bizley JK et al (2007) Physiological and behavioral studies of spatial coding in the auditory cortex. Hear Res 229(1–2):106–115
Siegel S, Vaquero JJ, Aloj L et al (1999) Initial results from a PET/Planar small animal imaging system. IEEE Trans on Nuclear Sci 46(3):571–575
Herráiz JL, España S, Vaquero JJ et al (2006) FIRST: Fast Iterative Reconstruction Software for (PET) tomography. Phys Med Biol 51:4547–4565
Jagoda EM, Vaquero JJ, Seidel J et al (2004) Experiment assessment of mass effects in the rat: implications for small animal PET imaging. Nucl Med Biol 31(6):771–779
Kruguer L, Saporta S, Swanson LW (1995) Photographic atlas of the rat brain: the cell and fiber architecture illustrated in three planes with stereotaxic coordinates. Cambridge University Press, New York, p 299
Rubins DJ, Melega WP, Lacan G et al (2003) Development and evaluation of an automated atlas-based image analysis method for microPET studies of the rat brain. Neuroimage 20(4):2100–2118
Pascau J, Gispert JD, Michaelides M et al (2008) Automated method for small-animal PET image registration with intrinsic validation. Mol Imaging Biol. doi:10.1007/s11307-008-0166-z
Pluim JP, Maintz JB, Viergever MA (2003) Mutual-information-based registration of medical images: a survey. IEEE Trans Med Imaging 22(8):986–1004
Friston KJ, Holmes AP, Worsley KJ et al (1995) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210
Dorey SE, Neveu MM, Burton LC et al (2003) The clinical features of albinism and their correlation with visual evoked potentials. Br J Ophthalmol 87(6):767–772
Neveu MM, Jeffery G, Burton LC et al (2003) Age-related changes in the dynamics of human albino visual pathways. Eur J Neurosci 18(7):1939–1949
Ilia M, Jeffery G (2000) Retinal cell addition and rod production depend on early stages of ocular melanin synthesis. J Comp Neurol 420(4):437–444
Friston KJ, Frith CD, Liddle PF et al (1991) Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 11(4):690–699
Turner R, Howseman A, Rees GE et al (1998) Functional magnetic resonance imaging of the human brain: data acquisition and analysis. Exp Brain Res 123(1–2):5–12
Ito K, Morrish PK, Rakshi JS et al (1999) Statistical parametric mapping with 18F-dopa PET shows bilaterally reduced striatal and nigral dopaminergic function in early Parkinson’s disease. J Neurol Neurosurg Psychiatry 66(6):754–758
Kiebel SJ, Friston KJ (2004) Statistical parametric mapping for event-related potentials: I. Generic considerations. Neuroimage 22(2):492–502
Nguyen PT, Holschneider DP, Maarek JM et al (2004) Statistical parametric mapping applied to an autoradiographic study of cerebral activation during treadmill walking in rats. Neuroimage 23(1):252–259
Holschneider DP, Yang J, Sadler TR et al (2006) Mapping cerebral blood flow changes during auditory-cued conditioned fear in the nontethered, nonrestrained rat. 29:1344–1358
Lee JS, Ahn SH, Lee DS et al (2005) Voxel-based statistical analysis of cerebral glucose metabolism in the rat cortical deafness model by 3D reconstruction of brain from autoradiographic images. Eur J Nucl Med Mol Imaging 32(6):696–701
Yang J, Sadler TR, Givrad TK et al (2007) Changes in brain functional activation during resting and locomotor states after unilateral nigrostriatal damage in rats. Neuroimage 36(3):755–773
Holschneider DP, Yang J, Guo Y et al (2007) Reorganization of functional brain maps after exercise training: importance of cerebellar–thalamic–cortical pathway. Brain Res 1184:96–107
Cross DJ, Flexman JA, Anzai Y et al (2008) Age-related decrease in axonal transport measured by MR imaging in vivo. Neuroimage 39(3):915–926
Easton N, Marshall F, Fone KC et al (2007) Differential effects of the D-and L-isomers of amphetamine on pharmacological MRI BOLD contrast in the rat. Psychopharmacology (Berl) 193(1):11–30
Easton N, Marshall F, Fone K et al (2007) Atomoxetine produces changes in cortico-basal thalamic loop circuits: assessed by phMRI BOLD contrast. Neuropharmacology 52(3):812–826
Littlewood CL, Jones N, O’Neill MJ et al (2006) Mapping the central effects of ketamine in the rat using pharmacological MRI. Psychopharmacology (Berl) 186(1):64–81
Lowe AS, Williams SC, Symms MR et al (2002) Functional magnetic resonance neuroimaging of drug dependence: naloxone-precipitated morphine withdrawal. Neuroimage 17(2):902–910
Schweinhardt P, Fransson P, Olson L et al (2003) A template for spatial normalisation of MR images of the rat brain. J Neurosci Methods 129(2):105–113
Casteels C, Vermaelen P, Nuyts J et al (2006) Construction and evaluation of multitracer small-animal PET probabilistic atlases for voxel-based functional mapping of the rat brain. J Nucl Med 47(11):1858–1866
van Kuyck K, Casteels C, Vermaelen P et al (2007) Motor-and food-related metabolic cerebral changes in the activity-based rat model for anorexia nervosa: a voxel-based microPET study. Neuroimage 35(1):214–221
Toga AW, Collins RC (1981) Metabolic response to optic centers to visual stimuli in the albino rat: anatomical and physiological considerations. J Comp Neurol 199(4):443–464
Soncrant TT, Holloway HW, Stipetic M et al (1988) Cerebral glucose utilization in rats is not altered by hind limb restraint or by femoral artery and vein cannulation. J Cereb Blood Flow Metab 8(5):720–726
Crane AM, Porrino LJ (1989) Adaptation of the quantitative 2-[14C]deoxyglucose method for use in freely moving rats. Brain Res 499(1):87–92
Linden R, Perry VH (1983) Retrograde and anterograde-transneuronal degeneration in the parabigeminal nucleus following tectal lesions in developing rats. J Comp Neurol 218(3):270–281
Cooper RM, Thurlow GA (1991) [2-14C]deoxyglucose uptake in rat visual system during flashing-diffuse and flashing-pattern stimulation over a 6 log range of luminance. Exp Neurol 113(1):79–84
Cooper RM, Allen K (1995) Metabolic activity in rat visual system during exposure to high and low intensities of patterned and diffuse light. Int J Neurosci 81(1–2):27–34
Thompson SK, von Kriegstein K, Deane-Pratt A et al (2006) Representation of interaural time delay in the human auditory midbrain. Nat Neurosci 9(9):1096–1098
Acknowledgments
This work was supported by grants from the Ministerio de Ciencia y Tecnología (TEC2004-07052), Ministerio de Sanidad y Consumo (CIBERsam CB07/09/0031 and Plan Nacional sobre Drogas 2007/043), Ministerio de Industria (CDTEAM Project), and Fundación de Investigación Médica Mutua Madrileña. We thank Alexandra de Francisco for her assistance with the PET studies, the Atomic, Molecular, and Nuclear Physics Department of the Universidad Complutense in Madrid for reconstructing the PET images, and the National Institute of Health for facilitating the piPET system.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soto-Montenegro, M.L., Vaquero, J.J., Pascau, J. et al. Detection of Visual Activation in the Rat Brain Using 2-deoxy-2-[18F]fluoro-d-glucose and Statistical Parametric Mapping (SPM). Mol Imaging Biol 11, 94–99 (2009). https://doi.org/10.1007/s11307-008-0179-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-008-0179-7