Abstract
Introduction
(S,S)-[11C]MeNER ((S,S)-2-(α-(2-[11C]methoxyphenoxy)benzyl)morpholine) is a positron emission tomography (PET) radioligand recently applied in clinical studies of norepinephrine transporters (NETs) in the human brain in vivo. In view of further assessment of the suitability of (S,S)-[11C]MeNER as a NET radioligand, its metabolism and the identity of the in vivo radiometabolites of (S,S)-[11C]MeNER are of great interest.
Materials and Methods
Thus, PET studies were used to measure brain dynamics of (S,S)-[11C]MeNER, and plasma reverse-phase radiochromatographic analysis was performed to monitor and quantify its rate of metabolism. Eighteen healthy human volunteers, five cynomolgus monkeys, and five rats were studied.
Results and Discussion
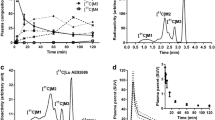
In human subjects, the plasma radioactivity representing (S,S)-[11C]MeNER decreased from 88 ± 5% at 4 min after injection to 82 ± 7% at 40 min, while a polar radiometabolite increased from 3 ± 3% to 16 ± 7% at the same time-points, respectively. A more lipophilic radiometabolite than (S,S)-[11C]MeNER decreased from 9 ± 5% at 4 min to 1 ± 2% at 40 min. In monkeys, plasma radioactivity representing (S,S)-[11C]MeNER decreased from 97 ± 2% at 4 min to 74 ± 7% at 45 min, with a polar fraction as the major radiometabolite. A more lipophilic radiometabolite than (S,S)-[11C]MeNER, constituted 3 ± 2% of radioactivity at 4 min and was not detectable later on. In rats, 17 ± 4% of plasma radioactivity was parent radioligand at 30 min with the remainder comprising mainly a polar radiometabolite. (S,S)-[11C]MeNER in rat brain and urine at 30 min after injection were 90% and 4%, respectively. On a brain regional level, parent radioligand ranged from 87.5 ± 3.9% (57.2 ± 14.2% SUV [standard uptake values, %injected radioactivity per mL multiplied with animal weight (in g)]; cerebellum) to 92.9 ± 1.8% (36.1 ± 4.7% SUV; striatum), with differential distribution of the radiometabolite in the cerebellum (6.7 ± 0.3% SUV) and the striatum (2.5 ± 0.3% SUV). Liquid chromatography-mass spectrometry analysis of rat urine identified a hydroxylation product of the methoxyphenoxy ring of (S,S)-MeNER as the main metabolite. In the brain, the corresponding main metabolite was the product from O-de-methylation of (S,S)-MeNER. PET measurements were performed in rats as well as in wild-type and P-gp-knock-out mice. In rats, the brain peak level of radioactivity was found to be very low (65%SUV). In mice, there was only a small difference in peak brain accumulation between P-gp knock-out and wild-type mice (145 vs. 125%SUV) with the following rank order of regional brain radioactivity: cerebellum × thalamus > cortical regions > striatum.
Conclusion
It can be concluded that radiometabolites of (S,S)-[11C]MeNER are of minor importance in rat and monkey brain imaging. The presence of a transient lipophilic radiometabolite in peripheral human plasma may induce complications with brain imaging, but its kinetics appear favorable in relation to the slow kinetics of (S,S)-[11C]MeNER in humans.




Similar content being viewed by others
References
Maziere B, Cantineau R, Coenen HH, Guillaume M, Halldin C, Luxen A, Loc’h C,Luthra SK (1993) Radiopharmaceuticals for positron emission tomography—methodological aspects. In G Stöcklin, VW Pike (ed) Kluwer Academic Press, Dordrecht, The Netherlands
Osman S, Lundkvist C, Pike VW, Halldin C, McCarron JA, Swahn CG, Farde L, Ginovart N, Luthra SK, Gunn RN, Bench CJ, Sargent PA, Grasby PM (1998) Characterisation of the appearance of radioactive metabolites in monkey and human plasma from the 5-HT1A receptor radioligand, [carbonyl-11C]WAY-100635–explanation of high signal contrast in PET and an aid to biomathematical modelling. Nucl Med Biol 25:215–223
Schou M, Halldin C, Sovago J, Pike VW, Gulyas B, Mozley PD, Johnson DP, Hall H, Innis RB, Farde L (2003) Specific in vivo binding to the norepinephrine transporter demonstrated with the PET radioligand, (S,S)-[11C]MeNER. Nucl Med Biol 30:707–714
Wilson A, Johnson D, Mozley P, Hussey D, Ginovart N, Nobrega J, Garcia A, Meyer J, Houle S (2003) Synthesis and in vivo evaluation of novel radiotracers for the in vivo imaging of the norepinephrine transporter. Nucl Med Biol 30:85–92
Ding YS, Lin KS, Garza V, Carter P, Alexoff D, Logan J, Shea C, Xu Y, King P (2003) Evaluation of a new norepinephrine transporter PET ligand in baboons, both in brain and peripheral organs. Synapse 50:345–352
Dostert P, Benedetti MS, Poggesi I (1997) Review of the pharmacokinetics and metabolism of reboxetine, a selective noradrenaline reuptake inhibitor. Eur Neuropsychopharmacol 7(Suppl 1):S23–S35, discussion S71–3
Halldin C, Swahn C-G, Farde L, Sedvall G (1995) Radioligand disposition and metabolism. In: Comar D (ed) PET for drug development and evaluation. Kluwer Academic Publishers, Dordrecht, pp 55–65
Lundberg J, Odano I, Olsson H, Halldin C, Farde L (2005) Quantification of 11C-MADAM binding to the serotonin transporter in the human brain. J Nucl Med 46:1505–1515
Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP et al (1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood–brain barrier and to increased sensitivity to drugs. Cell 77:491–502
Meneguz A, Fortuna S, Lorenzini P, Volpe MT (1991) Influence of urethane and ketamine on rat hepatic cytochrome P450 in vivo. Exp Toxicol Pathol 51:392–396
Wienkers LC, Allievi C, Hauer MJ, Wynalda MA (1999) Cytochrome P-450-mediated metabolism of the individual enantiomers of the antidepressant agent reboxetine in human liver microsomes. Drug Metab Dispos 27:1334–1340
Dischino D, Welch M, Kilbourn M, Raichle M (1983) Relationship between lipophilicity and brain extraction of C-11-labeled radiopharmaceuticals. J Nucl Med 24:1030–1083
Loscher W, Potschka H (2005) Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol 76:22–76
Andree B, Seneca N, Schou M, Mozley PD, Potter W, Farde L, Gulyas B, Halldin C (2004) Demonstration of selective norepinephrine transporter binding in human brain using PET and [C-11]MeNER. Eur J Nucl Med Mol Imaging 31:S208
Haradahira T, Zhang MR, Maeda J, Okauchi T, Kida T, Kawabe K, Sasaki S, Suhara T, Suzuki K (2001) A prodrug of NMDA/glycine site antagonist, L-703,717, with improved BBB permeability: 4-acetoxy derivative and its positron-emitter labeled analog. Chem Pharm Bull (Tokyo) 49:147–150
Smith HR, Beveridge TJ, Porrino LJ (2006) Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience 138:703–714
Donnan G, Kaczmarczyk S, Paxinos G, Chilco P, Kalnins R, Woodhouse D, Mendelsohn F (1991) Distribution of catecholaminergic uptake sites in human brain as determined by quantitative [3H]mazindol autoradiography. J Comp Neurol 304:419–434
Tejani-Butt S (1992) [3H]Nisoxetine: A radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. J Pharm Exp Ther 260:427–436
Zoghbi SS, Shetty HU, Ichise M, Fujita M, Imaizumi M, Liow JS, Shah J, Musachio JL, Pike VW, Innis RB (2006) PET imaging of the dopamine transporter with 18F-FECNT: a polar radiometabolite confounds brain radioligand measurements. J Nucl Med 47:520–527
Acknowledgments
This study was supported in part by the Intramural Research program of the National Institutes of Health (NIH), specifically the National Institute of Mental Health (NIMH). Dr. M. Schou also received support through the NIH-Karolinska Institutet (KI) graduate training partnership in neuroscience. The authors are grateful for the kind assistance of Arsalan Amir (KI), Jonathan Gourley (NIMH), Phong Truong (KI), and other members of the PET group at KI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schou, M., Zoghbi, S.S., Shetty, H.U. et al. Investigation of the Metabolites of (S,S)-[11C]MeNER in Humans, Monkeys and Rats. Mol Imaging Biol 11, 23–30 (2009). https://doi.org/10.1007/s11307-008-0175-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-008-0175-y