Abstract
Purpose
In this study, we introduce a methodology for preparing 18F-labeled Affibody protein, specifically 18F-Anti-HER2 dimeric Affibody (14 kDa), for in vivo imaging of HER2neu with positron emission tomography (PET).
Procedures
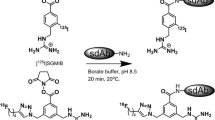
We have used 4-[18F]fluorobenzaldehyde as a synthon to prepare 18F-Anti-HER2 Affibody. Aminooxy-functionalized Affibody (Anti-HER2-ONH2) was incubated with 4-[18F]fluorobenzaldehyde in ammonium acetate buffer at pH 4 in the presence of methanol at 70°C for 15 min. The resulting 18F-labeled Affibody molecule was evaluated as a PET probe in xenograft models expressing HER2.
Results
We have successfully prepared 18F-Anti-HER2 dimeric Affibody (14 kDa), N-(4-[18F]fluorobenzylidine)oxime-Anti-HER2 Affibody, [18F]FBO-Anti-HER2, in 26–30% radiochemical yields (decay corrected). High-contrast small-animal PET images with relatively moderate tumor uptake (1.79 ± 0.40% ID/g) were observed for the 18F-Anti-HER2 Affibody.
Conclusion
Site-specific 18F-labeled Affibody against HER2 has been synthesized via chemoselective oxime formation between an aminooxy-functionalized Affibody and 18F-fluorobenzaldehyde. The results have implications for radiolabeling of other affibodies and macromolecules and should also be important for advancing Affibody imaging with PET.



Similar content being viewed by others
References
Gambhir SS (2002) Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer 2:683–693
Varagnolo L, Stokkel MPM, Mazzi U, Pauwels EK (2000) 18F-Labeled radiopharmaceuticals for PET in oncology, excluding FDG. J Nuc Med Biol 27:103–112
Gunneriussoin E, Nord K, Uhlen M, Nygren P (1999) Affinity maturation of a Taq DNA polymerase specific Affibody by helix shuffling. Protein Eng 12:873–878
Nord K, Gunneriusson E, Ringdahl J, Stahl S, Uhlen M, Nygren P-A (1997) Binding protein selected from combinatorial libraries on an alpha-helical bacterial receptor domain. Nat Biotechnol 15:772–777
Tran T, Engfeld T, Orlova A et al (2007) In vivo evaluation of cystine-based chelators for attachment of 99mTc to tumor-targeting Affibody molecules. Bioconjugate Chem 18:549–558
Orlaova A, Magnusson M, Eriksson TL et al (2006) Tumor imaging using a picomolar affinity HER2 binding Affibody molecule. Cancer Res 66:4339–4348
Orlova A, Tolmachev V, Pehrson R et al (2007) Synthetic Affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res 67:21788–2186
Poethko T, Schottelius M, Thumshirn G et al (2004) Two-step methodology for high yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J Nucl Med 45:892–902
Toyokuni T, Walsh JC, Dominguez A, Phelps ME, Barrio JR, Gambhir SS, Satyamurthy N (2003) Synthesis of a new hetrobifunctional linker, N-[4-(aminooxy)butyl]maleimide, for facile access to a thiol-reactive 18F-labeling agent. Bioconjug Chem 14:1253–1259
Berndt M, Pietzsch J, Wuest F (2007) Labeling of low-density lipoproteins 18F-labeled thiol-reactive reagent N-[6-[18F]fluorobenzylidine)aminooxyhexyl]maeimide. Nucl Med Biol 34:5–25
Cai W, Zhang X, Wu Y, Chen X (2006) A thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido)ethyl]maleimide, and synthesis of RGD peptides-based tracer for PET imaging of αvβ3 integrine expression. J Nucl Med 47:172–180
Calzadilla M, Cordovoa T, Malpica A (1990) Effect of structure on reactivity in oxime formation of benzaldehyde. J Phys Org Chem 12:408–712
Rosenbeg S, Silver SM, Sayer JM, Jacks WP (1974) Evidence for two concurrent mechanisms and a kinetically significant proton transfer in avid-catalyzed O-methyloxime formation. J Am Chem Soc 96:798
Hang HC, Bertozzi CR (2001) Chemoselective approaches to glycoprotein assembly. Acc Chem Res 34:727–736
Cornish VW, Hahn KM, Schultz PG (1996) Site-specific protein modification using a ketone handle. J Am Chem Soc 118:8150–8151
Gilmore JM, Scheck RA, Esser-Kahn AP, Joshi NA, Francis MB (2006) N-terminal protein modification through a biomimetic transamination reaction. Angew Chem Int Ed 45:5307–5311
Ruiz-Chica AJ, Medina MA, Sanchez-Jimenez F, Ramirez FJ (2004) On the interpretation of Raman spectra of 1-aminooxy-spermine/DNA complex. Nucleic Acids Res 32:579–589
Yu D, Wolf JK, Scanlon M et al (1993) Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Research 53:891–898
Engfeldt T, Tran T, Orlova A et al (2007) (99m)Tc-chelator engineering to improve tumor targeting properties of a HER2-specific Affibody molecule. Eur J Nucl Med Mol Imaging 34:1843–1853
Acknowledgment
This work was supported, in part, by Medical Diagnostics, GE Healthcare, National Cancer Institute (NCI) Small Animal Imaging Resource Program (SAIRP) grant R24 CA93862, and NCI In Vivo Cellular Molecular Imaging Center (ICMIC) grant P50 CA114747 (SSG). We also thank Dr. David Dick for [18F] production, Dr. Frederick T. Chin for modification of a GE TRACERlab FX-FN synthetic module for radiosynthesis of 4-[18F]FBA, and Dr. Alan Cuthbertson and Dr. Alex Gibson of GE Healthcare for their review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Namavari, M., Padilla De Jesus, O., Cheng, Z. et al. Direct Site-Specific Radiolabeling of an Affibody Protein with 4-[18F]Fluorobenzaldehyde via Oxime Chemistry. Mol Imaging Biol 10, 177–181 (2008). https://doi.org/10.1007/s11307-008-0142-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-008-0142-7