Abstract
Purpose
The aim of this study was to establish the procedures for 3D voxel-based statistical analysis of 2-deoxy-2-[18F]fluro-d-glucose-positron emission tomography (FDG-PET) images of a cat’s brain obtained using a small animal-dedicated PET system and to assess the utility of this approach in investigating the cerebral glucose metabolism in an animal model of cortical deafness.
Procedures
This study compared several different strategies for the spatial processing of PET data acquired twice from eight cats before and after inducing deafness in terms of the comparability of the statistical analysis results to the established pattern of the cerebral glucose metabolic changes in the deaf animals.
Results
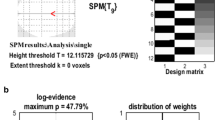
The accuracy of the spatial preprocessing procedures and the statistical significance of the comparison were improved by removing the background activities outside the brain regions. The use of the spatial normalization parameters obtained from the mean image of the realigned data set for individual data also helped improve the statistical significance of the paired t testing. It was also found that an adjustment of the registration options was also important for increasing the precision of the realignment.
Conclusions
A method for voxel-based analysis of the PET data of a cat’s brain was optimized. The results demonstrated the high localization accuracy and specificity of this method, which is expected to be useful for examining the brain PET data of medium-sized animals such as cats.





Similar content being viewed by others
References
Ito J, Sakakibara J, Iwasaki Y, Yonekura Y (1993) Positron emission tomography of auditory sensation in deaf patients and patients with cochlear implants. Ann Otol Rhinol Laryngol 102:797–801
Catalan-Ahumada M, Deggouj N, De Volder A et al (1993) High metabolic activity demonstrated by positron emission tomography in human auditory cortex in case of deafness of early onset. Brain Res 623:287–292
Deggouj N, Devolder A, Catalan M et al (1995) Positron emission tomography in deaf patients at rest. Adv Otorhinolaryngol 50:31–37
Lee DS, Lee JS, Oh SH et al (2001) Cross-modal plasticity and cochlear implants. Nature 409:149–150
Lee JS, Lee DS, Oh SH et al (2003) PET evidence of neuroplasticity in adult auditory cortex of postlingual deafness. J Nucl Med 44:1435–1439
Bavelier D, Neville HJ (2002) Cross-modal plasticity: where and how? Nat Rev Neurosci 3:443–452
Cordes M, Wszolek ZK (2003) Deafness and cerebral plasticity. J Nucl Med 44:1440–1442
Cohen LG, Celnik P, Pascual-Leone A et al (1997) Functional relevance of cross-modal plasticity in blind humans. Nature 389:180–183
Nishimura H, Hashikawa K, Doi K et al (1999) Sign language ‘heard’ in the auditory cortex. Nature 397:116
Ahn SH, Oh SH, Lee JS et al (2004) Changes of 2-deoxyglucose uptake in the rat auditory pathway after bilateral ablation of the cochlea. Hear Res 196:33–38
Lee JS, Ahn SH, Lee DS et al (2005) Voxel-based statistical analysis of cerebral glucose metabolism in the rat cortical deafness model by 3D reconstruction of brain from autoradiographic images. Eur J Nucl Med Mol Imaging 32:696–701
Kornblum HI, Araujo DM, Annala AJ et al (2000) In vivo imaging of neuronal activation and plasticity in the rat brain by high resolution positron emission tomography (microPET). Nat Biotechnol 18:655–660
Moore TH, Osteen TL, Chatziioannou TF, Hovda DA, Cherry TR (2000) Quantitative assessment of longitudinal metabolic changes in vivo after traumatic brain injury in the adult rat using FDG-microPET. J Cereb Blood Flow Metab 20:1492–1501
Mir HM, Tatsukawa KJ, Carmichael ST, Chesselet MF, Kornblum HI (2004) Metabolic correlates of lesion-specific plasticity: an in vivo imaging study. Brain Res 1002:28–34
Kim JS, Lee JS, Im KC et al (2007) Performance measurement of the microPET Focus 120 scanner. J Nucl Med 48:1527–1535
Tai YC, Ruangma A, Rowland D et al (2005) Performance evaluation of the microPET focus: a third-generation microPET scanner dedicated to animal imaging. J Nucl Med 46:455–463
Schafers KP, Reader AJ, Kriens M et al (2005) Performance evaluation of the 32-module quadHIDAC small-animal PET scanner. J Nucl Med 46:996–1004
Xu SA, Shepherd RK, Chen Y, Clark GM (1993) Profound hearing loss in the cat following the single co-administration of kanamycin and ethacrynic acid. Hear Res 70:205–215
Friston KJ, Ashburner J, Frith CD et al (1995) Spatial registration and normalization of images. Human Brain Mapping 3:165–189
Ashburner J, Friston KJ (1999) Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7:254–266
Lee JS, Park KS, Lee DS et al (2005) Development and applications of a software for Functional Image Registration (FIRE). Comput Methods Programs Biomed 78:157–164
Reinoso-Suarez F (1961) Topographisher Hirnatlas der Katze. Merck, Darmstadt, Germany
Heiss WD, Graf R, Lottgen J et al (1997) Repeat positron emission tomographic studies in transient middle cerebral artery occlusion in cats: residual perfusion and efficacy of postischemic reperfusion. J Cereb Blood Flow Metab 17:388–400
Nariai T, Shimada Y, Ishiwata K et al (2003) PET imaging of adenosine A1 receptors with 11C-MPDX as an indicator of severe cerebral ischemic insult. J Nucl Med 44:1839–1844
Ginovart N, Wilson AA, Houle S, Kapur S (2004) Amphetamine pretreatment induces a change in both D2-Receptor density and apparent affinity: a [11C]raclopride positron emission tomography study in cats. Biol Psychiatry 55:1188–1194
Surti S, Karp JS, Perkins AE et al (2005) Imaging performance of A-PET: a small animal PET camera. IEEE Trans Med Imaging 24:844–852
Rafecas M, Boning G, Pichler BJ et al (2003) Inter-crystal scatter in a dual layer, high resolution LSO-APD positron emission tomograph. Phys Med Biol 48:821–848
Matsumura A, Mizokawa S, Tanaka M et al (2003) Assessment of microPET performance in analyzing the rat brain under different types of anesthesia: comparison between quantitative data obtained with microPET and ex vivo autoradiography. Neuroimage 20:2040–2050
Kang E, Lee DS, Lee JS et al (2003) Developmental hemispheric asymmetry of interregional metabolic correlation of the auditory cortex in deaf subjects. Neuroimage 19:777–783
Nguyen PT, Holschneider DP, Maarek JM et al (2004) Statistical parametric mapping applied to an autoradiographic study of cerebral activation during treadmill walking in rats. Neuroimage 23:252–259
Acknowledgements
This work was supported by grants from the Seoul National University Hospital (04-2007-037) and the Basic Research Program of the Korea Science and Engineering Foundation (R01-2006-000-10296-0), Brain Research Center of the 21st Century Frontier Research Program (M103KV010017-07K2201-01710), and Basic Atomic Energy Research Institute Program (M20508050002-05B0805-00210, 2007-01022) funded by the Ministry of Science and Technology. The authors appreciate the experimental support of Kim Seung Jeong, Lee Ho Sun, and Park Soo-Ah and software application support of David L. Bailey (Siemens Molecular Imaging).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.S., Lee, J.S., Park, MH. et al. Assessment of Cerebral Glucose Metabolism in Cat Deafness Model: Strategies for Improving the Voxel-Based Statistical Analysis for Animal PET Studies. Mol Imaging Biol 10, 154–161 (2008). https://doi.org/10.1007/s11307-008-0140-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-008-0140-9