Abstract
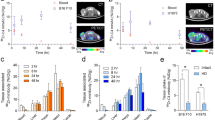
The bioluminescent protein Gaussia luciferase (GLuc) was fused to an anti-carcinoembryonic antigen (CEA) antibody fragment, the diabody, for in vivo optical tumor imaging. A 15-amino acid N-terminal truncation (GLΔ15) resulted in a brighter protein. Fusions of the anti-CEA diabody to full-length GLuc and GLΔ15 retained high affinity for the antigen, emitted light, and exhibited excellent enzymatic stability. In vivo optical imaging of tumor-bearing mice demonstrated specific targeting of diabody-GLΔ15 to CEA-positive xenografts, with a tumor/background ratio of 3.8 ± 0.4 at four hours after tail-vein injection, compared to antigen-negative tumors at 1.3 ± 0.1 (p = 0.001). MicroPET imaging using 124I-diabody-GLΔ15 demonstrated specific uptake in the CEA-positive tumor (2.6% ID [injected dose]/g) compared to the CEA-negative tumor (0.4% ID/g) at 21 hours. Although further optimization of this fusion protein may be needed to improve in vivo performance, the diabody-GLΔ15 is a promising optical imaging probe for tumor detection in vivo.







Similar content being viewed by others
References
Zhao H, Doyle TC, Coquoz O, et al. (2005) Emission spectra of bioluminescent reporters and interaction with mammalian tissue determine the sensitivity of detection in vivo. J Biomed Opt 10(4):41210
Loening AM, Fenn TD, Wu AM, Gambhir SS (2006) Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel 19(9):391–400
Holliger P, Prospero T, Winter G (1993) Diabodies: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA 90(14):6444–6448
Wu AM, Williams LE, Zieran L, et al. (1999) Anti-carcinoembryonic antigen (CEA) diabody for rapid tumor targeting and imaging. Tumor Targeting 4:47–58
Sundaresan G, Yazaki PJ, Shively JE, et al. (2003) 124I-labeled engineered anti-CEA minibodies and diabodies allow high-contrast, antigen-specific small-animal PET imaging of xenografts in athymic mice. J Nucl Med 44(12):1962–1969
Lorenz WW, Cormier MJ, O’Kane DJ, et al. (1996) Expression of the Renilla reniformis luciferase gene in mammalian cells. J Biolumin Chemilumin 11(1):31–37
Massoud TF, Gambhir SS (2003) Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev 17(5):545–580
Shah K, Bureau E, Kim DE, et al. (2005) Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol 57(1):34–41
Gross S, Piwnica-Worms D (2005) Spying on cancer: molecular imaging in vivo with genetically encoded reporters. Cancer Cell 7(1):5–15
Bhaumik S, Gambhir SS (2002) Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci USA 99(1):377–382
Bhaumik S, Lewis XZ, Gambhir SS (2004) Optical imaging of Renilla luciferase, synthetic Renilla luciferase, and firefly luciferase reporter gene expression in living mice. J Biomed Opt 9(3):578–586
Matthews JC, Hori K, Cormier MJ (1977) Substrate and substrate analogue binding properties of Renilla luciferase. Biochemistry 16(24):5217–5220
Verhaegent M, Christopoulos TK (2002) Recombinant Gaussia luciferase. Overexpression, purification, and analytical application of a bioluminescent reporter for DNA hybridization. Anal Chem 74(17):4378–4385
Tannous BA, Kim DE, Fernandez JL, Weissleder R, Breakefield XO (2005) Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther 11(3):435–443
Venisnik KM, Olafsen T, Loening AM, et al. (2006) Bifunctional antibody-Renilla luciferase fusion protein for in vivo optical detection of tumors. Protein Eng Des Sel 19(10):453–460
Takkinen K, Laukkanen ML, Sizmann D, et al. (1991) An active single-chain antibody containing a cellulase linker domain is secreted by Escherichia coli. Protein Eng 4(7):837–841
Yazaki PJ, Shively L, Clark C, et al. (2001) Mammalian expression and hollow fiber bioreactor production of recombinant anti-CEA diabody and minibody for clinical applications. J Immunol Methods 253(1–2):195–208
Wu AM, Yazaki PJ, Tsai S, et al. (2000) High-resolution microPET imaging of carcinoembryonic antigen-positive xenografts by using a copper-64-labeled engineered antibody fragment. Proc Natl Acad Sci USA 97(15):8495–8500
Kenanova V, Olafsen T, Crow DM, et al. (2005) Tailoring the pharmacokinetics and positron emission tomography imaging properties of anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments. Cancer Res 65(2):622–631
Chow PL, Rannou FR, Chatziioannou AF (2005) Attenuation correction for small animal PET tomographs. Phys Med Biol 50(8):1837–1850
Defrise M, Kinahan PE, Townsend DW, et al. (1997) Exact and approximate rebinning algorithms for 3-D PET data. IEEE Trans Med Imaging 16(2):145–158
Kinahan PE, Rogers JG (1989) Analytic 3D image reconstruction using all detected events. IEEE Trans Nucl Sci 36(1):964–968
Loening AM, Gambhir SS (2003) AMIDE: a free software tool for multimodality medical image analysis. Molecular Imaging 2(3):131–137
Acknowledgments
The authors wish to thank Dr. Arion Chatziioannou, Dr. David Stout, Waldemar Lladno, and Judy Edwards for assistance with the microPET imaging studies. We appreciate the advice and assistance of Vania Kenanova, Karl Bauer, and Felix Bergara during various stages of the work. We thank Dr. Bruce Bryan for providing plasmids and proteins, and also appreciate the insights of Dr. Bruce Bryan, Dr. Byron Ballou, and Dr. Chris Szent-Gyorgi on the repeated sequence and possible signal peptide in the GLuc gene. This work was supported in part by NIH Grants T32 GM 08652 (K.M.V.), P01 CA 43904 (T.O., A.M.W.), R24 CA92865 (S.S.G.), R01 CA082214 (S.S.G.). A.M.W. is a member of the Jonsson Comprehensive Cancer Center (NIH P30 CA 16042).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venisnik, K.M., Olafsen, T., Gambhir, S.S. et al. Fusion of Gaussia Luciferase to an Engineered Anti-carcinoembryonic Antigen (CEA) Antibody for In Vivo Optical Imaging. Mol Imaging Biol 9, 267–277 (2007). https://doi.org/10.1007/s11307-007-0101-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-007-0101-8