Abstract
Purpose
The aim of the study is to assess the feasibility of imaging specific activity of myeloperoxidase (MPO), a leukocyte enzyme with important roles in inflammation and atherosclerosis, by single photon emission computed tomography (SPECT) using a novel 67Ga-labeled radiotracer obtained by conjugating desferrioxamine (DF) and hydroxyindolyl acetic acid in vivo.
Materials and Methods
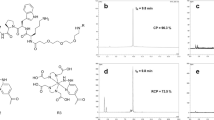
A reducing substrate of MPO (I) was synthesized by reacting commercially available DF with 2-(5-hydroxy-1H-indol-3-yl) acetic acid in the presence of a coupling agent [dicyclohexyl carbodiimide (DCC)]. The chelating unit was labeled with 67Ga, and its interaction with MPO was characterized using MALDI-TOF and UV–vis. Mice with Matrigel implants containing human MPO were used to model diseased tissues rich in MPO. Three hours after the injection of 67Ga–I, SPECT/computed tomography (CT) imaging was performed on a high-resolution Gamma Medica X-SPECT system. Biodistribution studies were performed six hours after the injection of the radiotracer.
Results
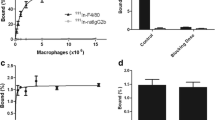
The feasibility of compound I oligomerization in the presence of MPO and MPO-mediated cross-linking with proteins was initially confirmed in vitro. In vivo, a 2.7-fold increase in target-to-muscle ratio could be measured in MPO-containing Matrigel implants in mice. Biodistribution experiments demonstrated a 60% increase of radioactivity in MPO-containing vs. control (contralateral) Matrigel implants.
Conclusion
67Ga–I can be used to image MPO activity in a model system. The accumulation mechanism is based on a differential pharmacokinetics because of the size increase resulting from 67Ga–I interaction with the target enzyme.








Similar content being viewed by others
References
Blasberg RG, Gelovani J (2002) Molecular-genetic imaging: A nuclear medicine-based perspective. Mol Imaging 1:280–300
Massoud TF, Gambhir SS (2003) Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes Dev 17:545–580
Bogdanov A Jr, Weissleder R (2002) In vivo imaging of gene delivery and expression. Trends Biotechnol 20:11S–18S
Rausch PG, Pryzwansky KB, Spitznagel JK (1978) Immunocytochemical identification of azurophilic and specific granule markers in the giant granules of Chediak–Higashi neutrophils. N Engl J Med 298:693–698
Nicholls S, Hazen S (2005) Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 25:1102–1111
Daugherty A, Dunn JL, Rateri DL, Heinecke JW (1994) Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 94:437–444
Nagra RM, Becher B, Tourtellotte WW, et al. (1997) Immunohistochemical and genetic evidence of myeloperoxidase involvement in multiple sclerosis. J Neuroimmunol 78:97–107
Heuther G, Reimer A, Schmidt F, Schuff-Werner P, Brudny MM (1990) Oxidation of the indole nucleus of 5 hydroxytryptamine and formation of dimers in the presence of peroxidase and H2O2. J Neural Transm Suppl 32:249–257
Heinecke JW, Li W, Francis GA, Goldstein JA (1993) Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest 91:2866–2872
Chen JW, Pham W, Weissleder R, Bogdanov A (2004) Human myeloperoxidase: A potential target for molecular MR imaging in atherosclerosis. J Magn Reson Med 52:1021–1028
Zhang C, Yang J, Jacobs JD, Jennings LK (2003) Interaction of myeloperoxidase with vascular NAD(P)H oxidase-derived reactive oxygen species in vasculature: Implications for vascular diseases. Am J Physiol, Heart Circ Physiol 285:H2563–H2572
Bergt C, Pennathur S, Fu X, et al. (2004) The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA 101:13032–13037
Hershko CM, Link GM, Konijn AM, Cabantchik ZI (2005) Iron chelation therapy. Curr Hematol Rep 4:110–116
Wag S, Robert JL, Mathias CJ, Green MA, Low PS (1996) Synthesis, purification, and tumor cell uptake of 67Ga–desferrioxamine–folate, a potential radiopharmaceutical for tumor imaging. Bioconjug Chem 7:56–62
Mathias CJ, Lewis MR, Reichert DE, Laforest R, Sharp TL, Lewis JS, Yang, Z-F, Waters DJ, Snyder PW, Low PS, Welch MJ, Green MA (2003) Preparation of 66Ga- and 68Ga-labelled Ga(III)–desferrio-xamine–folate as potential folate-receptor-targeted PET radiopharmaceuticals. Nucl Med Biol 30:725–731
Reichert DE, Lewis JS, Anderson CJ (1999) Metal complexes as diagnostic tools. Coord Chem Rev 184:3–66
Anderson CJ, Welch M (1999) Radiometal-labeled agents (non-technetium) for diagnostic imaging. Chem Rev 99:2219–2234
Weiner R, Hoffer PB, Thakur ML (1981) Lactoferrin: Its role as a Ga-67-binding protein in polymorphonuclear leukocytes. J Nucl Med 22:32–37
Tsan MF (1985) Mechanism of gallium-67 accumulation in inflammatory lesions. J Nucl Med 26:88–92
Weiner R (1996) The mechanism of 67Ga localization in malignant disease. Nucl Med Biol 23:745–751
Tien M (1999) Myeloperoxidase-catalyzed oxidation of tyrosine. Arch Biochem Biophys 367:61–66
McCormick ML, Gaut JP, Lin TS, Britigan BE, Buettner GR, Heinecke JW (1998) Electron paramagnetic resonance detection of free tyrosyl radical generated by myeloperoxidase, lactoperoxidase, and horseradish peroxidase. J Biol Chem 273:32030–32037
Jantschko W, Furtmuller PG, Allegra M, Livrea MA, Jakopitsch C, Regelsberger G, Obinger C (2002) Redox intermediates of plant and mammalian peroxidases: A comparative transient-kinetic study of their reactivity toward indole derivatives. Arch Biochem Biophys 398:12–22
Querol M, Chen J, Weissleder R, Bogdanov A (2005) DTPA-bisamide-based MR sensor agents for peroxidase imaging. Org Lett 7:1719–1722
Acknowledgments
Partial support for this study was provided by the RSNA Research and Education Foundation (JWC) and the NIH [P50-CA86355, R01-HL078641 (RW), and R01 EB000858 (AB)].
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Querol and J. W. Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Querol Sans, M., Chen, J.W., Weissleder, R. et al. Myeloperoxidase Activity Imaging Using 67Ga Labeled Substrate. Mol Imaging Biol 7, 403–410 (2005). https://doi.org/10.1007/s11307-005-0020-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-005-0020-5