Abstract
Introduction
Nearly all the enzymes that mediate the metabolism of polyunsaturated fatty acids (PUFAs) are present in the kidney. However, the correlation of renal dysfunction with PUFAs metabolism in uremic patients remains unknown.
Objectives
To test whether the alterations in the metabolism of PUFAs reflect the renal dysfunction in uremic patients.
Methods
LC–MS/MS-based oxylipin profiling was conducted for the plasma samples from the uremic patients and controls. The data were analyzed by principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA). The receiver operating characteristic (ROC) curves and the correlation of the estimated glomerular filtration rate (eGFR) with the key markers were evaluated. Furthermore, qPCR analysis of the whole blood cells was conducted to investigate the possible mechanisms. In addition, a 2nd cohort was used to validate the findings from the 1st cohort.
Results
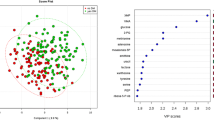
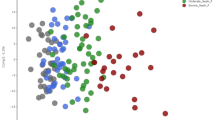
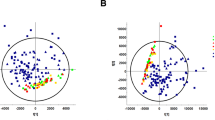
The plasma oxylipin profile distinguished the uremic patients from the controls successfully by using both PCA and OPLS-DA models. 5,6-Dihydroxyeicosatrienoic acid (5,6-DHET), 5-hydroxyeicosatetraenoic acid (5-HETE), 9(10)-epoxyoctadecamonoenoic acid [9(10)-EpOME] and 12(13)-EpOME were identified as the key markers to discriminate the patients from controls. The excellent predictive performance of these four markers was validated by ROC analysis. The eGFR significantly correlated with plasma levels of 5,6-DHET and 5-HETE positively but with plasma 9(10)-EpOME and 12(13)-EpOME negatively. The changes of these markers may account for the inactivation of cytochrome P450 2C18, 2C19, microsome epoxide hydrolase (EPHX1), and 5-lipoxygenase in the patients.
Conclusion
The alterations in plasma metabolic profile reflect the renal dysfunction in the uremic patients.



Similar content being viewed by others
Abbreviations
- AKI:
-
Acute kidney injury
- ARA:
-
Arachidonic acid
- CKD:
-
Chronic kidney disease
- COX:
-
Cyclooxygenase
- CYP:
-
Cytochrome P450
- EDTA:
-
Ethylenediaminetetraacetic acid
- EDP:
-
Epoxydocosapentaenoic acid
- EET:
-
Epoxyeicosatrienoic acid
- eGFR:
-
Estimated glomerular filtration rate
- DHDPE:
-
Dihydroxydocosapentaenoic acid
- DHET:
-
Dihydroxyeicosatrienoic acid
- DHETE:
-
Dihydroxyeicosatetraenoic acid
- DHOME:
-
Dihydroxy octadecamonoenoic acid
- EH:
-
Epoxide hydrolase
- EpETE:
-
Epoxyeicosatetraenoic acid
- EpOME:
-
Epoxyoctadecamonoenoic acid
- EPA:
-
Eicosapentaenoic acid
- ESRD:
-
End stage renal disease
- HETE:
-
Hydroxyeicosatetrasanoic acid
- HODE:
-
Hydroxyoctadecadienoic acid
- HOTrE:
-
Hydroxyoctadecatrienoic acid
- LA:
-
Linoleic acid
- LOX:
-
Lipoxygenase
- LSM:
-
Lipid signaling mediator
- OPLS-DA:
-
Orthogonal partial least squares-discriminant analysis
- oxo-ODE:
-
Oxo-octadecadienoic acid
- oxo-ETE:
-
Oxo-eicosatetraenoic acid
- PCA:
-
Principal component analysis
- PG:
-
Prostaglandin or prostacyclin
- PUFA:
-
Polyunsaturated fatty acid
- ROC:
-
Receiver operating characteristic
- TX:
-
Thromboxane
- VIP:
-
Variable importance for projection
References
Bautista-Garcia, P., Sanchez-Lozada, L. G., Cristobal-Garcia, M., Tapia, E., Soto, V., Avila-Casado, M. C., et al. (2006). Chronic inhibition of NOS-2 ameliorates renal injury, as well as COX-2 and TGF-beta 1 overexpression in 5/6 nephrectomized rats. Nephrology Dialysis Transplantation, 21, 3074–3081. https://doi.org/10.1093/ndt/gfl444.
Bettaieb, A., Koike, S., Chahed, S., Zhao, Y., Bachaalany, S., Hashoush, N., et al. (2017). Podocyte-specific soluble epoxide hydrolase deficiency in mice attenuates acute kidney injury. FEBS Journal, 284, 1970–1986. https://doi.org/10.1111/febs.14100.
Bhandari, S. (2006). How to measure renal function in clinical practice: Age affects estimated glomerular filtration rate. BMJ, 333, 918. https://doi.org/10.1136/bmj.333.7574.918.
Certikova Chabova, V., Kujal, P., Skaroupkova, P., Varnourkova, Z., Vackova, S., Huskova, Z., et al. (2018). Combined inhibition of soluble epoxide hydrolase and renin-angiotensin system exhibits superior renoprotection to renin-angiotensin system blockade in 5/6 nephrectomized ren-2 transgenic hypertensive rats with established chronic kidney disease. Kidney and Blood Pressure Research, 43, 329–349. https://doi.org/10.1159/000487902.
Cho, H., Hamza, A., Zhan, C. G., & Tai, H. H. (2005). Key NAD+-binding residues in human 15-hydroxyprostaglandin dehydrogenase. Archives of Biochemistry and Biophysics, 433, 447–453. https://doi.org/10.1016/j.abb.2004.09.036.
Dai, Y., Iwanaga, K., Lin, Y. S., Hebert, M. F., Davis, C. L., Huang, W., et al. (2004). In vitro metabolism of cyclosporine A by human kidney CYP3A5. Biochemical Pharmacology, 68, 1889–1902. https://doi.org/10.1016/j.bcp.2004.07.012.
DeLong, E. R., DeLong, D. M., & Clarke-Pearson, D. L. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics, 44, 837–845.
Deng, B. Q., Luo, Y., Kang, X., Li, C. B., Morisseau, C., Yang, J., et al. (2017). Epoxide metabolites of arachidonate and docosahexaenoate function conversely in acute kidney injury involved in GSK3beta signaling. Proceedings of the National Academy of Sciences of the USA, 114, 12608–12613. https://doi.org/10.1073/pnas.1705615114.
Dennis, E. A., & Norris, P. C. (2015). Eicosanoid storm in infection and inflammation. Nature Reviews Immunology, 15, 511–523. https://doi.org/10.1038/nri3859.
Diederich, S., Hanke, B., Bahr, V., & Oelkers, W. (1996). The metabolism of 9 alpha-fluorinated steroids in the human kidney. Endocrine Research, 22, 803–810.
Dobrian, A. D., Lieb, D. C., Cole, B. K., Taylor-Fishwick, D. A., Chakrabarti, S. K., & Nadler, J. L. (2011). Functional and pathological roles of the 12-and 15-lipoxygenases. Progress in Lipid Research, 50, 115–131. https://doi.org/10.1016/j.plipres.2010.10.005.
Esser-von Bieren, J. (2017). Immune-regulation and -functions of eicosanoid lipid mediators. Biological Chemistry, 398, 1177–1191. https://doi.org/10.1515/hsz-2017-0146.
Fujihara, C. K., Antunes, G. R., Mattar, A. L., Andreoli, N., Malheiros, D. M. A. C., Noronha, I. L., et al. (2003). Cyclooxygenase-2 (COX-2) inhibition limits abnormal COX-2 expression and progressive injury in the remnant kidney. Kidney International, 64, 2172–2181. https://doi.org/10.1046/j.1523-1755.2003.00319.x.
Gooch, K., Culleton, B. F., Manns, B. J., Zhang, J. G., Alfonso, H., Tonelli, M., et al. (2007). NSAID use and progression of chronic kidney disease. American Journal of Medicine. https://doi.org/10.1016/j.amjmed.2006.02.015.
Harris, R. C. (2002). Cyclooxygenase-2 and the kidney: Functional and pathophysiological implications. Journal of Hypertension, 20, S3–S9.
Hoxha, M. (2017). A systematic review on the role of eicosanoid pathways in rheumatoid arthritis. Advances in Medical Sciences, 63, 22–29. https://doi.org/10.1016/j.advms.2017.06.004.
Imig, J. D., Walsh, K. A., Khan, M. A. H., Nagasawa, T., Cherian-Shaw, M., Shaw, S. M., et al. (2012). Soluble epoxide hydrolase inhibition and peroxisome proliferator activated receptor gamma agonist improve vascular function and decrease renal injury in hypertensive obese rats. Experimental Biology and Medicine, 237, 1402–1412. https://doi.org/10.1258/ebm.2012.012225.
Jia, Z. J., Wang, H. P., & Yang, T. X. (2012). Microsomal prostaglandin E synthase 1 deletion retards renal disease progression but exacerbates anemia in mice with renal mass reduction. Hypertension, 59, 122–299. https://doi.org/10.1161/Hypertensionaha.111.178897.
Jung, O., Jansen, F., Mieth, A., Barbosa-Sicard, E., Pliquett, R. U., Babelova, A., et al. (2010). Inhibition of the soluble epoxide hydrolase promotes albuminuria in mice with progressive renal disease. PLoS ONE. https://doi.org/10.1371/journal.pone.0011979
Kamata, M., Hosono, K., Fujita, T., Kamata, K., & Majima, M. (2015). Role of cyclooxygenase-2 in the development of interstitial fibrosis in kidneys following unilateral ureteral obstruction in mice. Biomedicine & Pharmacotherapy, 70, 174–180. https://doi.org/10.1016/j.biopha.2015.01.010.
Kato, Y., Fuchi, N., Nishimura, Y., Watanabe, A., Yagi, M., Nakadera, Y., et al. (2014). Discovery of 1-oxa-4,9-diazaspiro[5.5]undecane-based trisubstituted urea derivatives as highly potent soluble epoxide hydrolase inhibitors and orally active drug candidates for treating of chronic kidney diseases. Bioorganic & Medicinal Chemistry Letters, 24, 565–570. https://doi.org/10.1016/j.bmcl.2013.12.020.
Kim, J., Imig, J. D., Yang, J., Hammock, B. D., & Padanilam, B. J. (2014). Inhibition of soluble epoxide hydrolase prevents renal interstitial fibrosis and inflammation. American Journal of Physiology-Renal Physiology, 307, F971–F980. https://doi.org/10.1152/ajprenal.00256.2014.
Kim, J., Yoon, S. P., Toews, M. L., Imig, J. D., Hwang, S. H., Hammock, B. D., et al. (2015). Pharmacological inhibition of soluble epoxide hydrolase prevents renal interstitial fibrogenesis in obstructive nephropathy. American Journal of Physiology-Renal Physiology, 308, F131–F139. https://doi.org/10.1152/ajprenal.00531.2014.
Korotkova, M., & Jakobsson, P. J. (2014). Persisting eicosanoid pathways in rheumatic diseases. Nature Reviews Rheumatology, 10, 229–241. https://doi.org/10.1038/nrrheum.2014.1.
Ladda, M. A., & Goralski, K. B. (2016). The effects of CKD on cytochrome P450-mediated drug metabolism. Advances in Chronic Kidney Disease, 23, 67–75. https://doi.org/10.1053/j.ackd.2015.10.002.
Lee, J. P., Yang, S. H., Lee, H. Y., Kim, B., Cho, J. Y., Paik, J. H., et al. (2012). Soluble epoxide hydrolase activity determines the severity of ischemia-reperfusion injury in kidney. PLoS ONE, 7, e37075. https://doi.org/10.1371/journal.pone.0037075.
Lenihan-Geels, G., Bishop, K. S., & Ferguson, L. R. (2016). Cancer risk and eicosanoid production: Interaction between the protective effect of long chain omega-3 polyunsaturated fatty acid intake and genotype. Journal of Clinical Medicine. https://doi.org/10.3390/jcm5020025.
Liang, Y. X., Jing, Z. Y., Deng, H., Li, Z. Q., Zhuang, Z., Wang, S., et al. (2015). Soluble epoxide hydrolase inhibition ameliorates proteinuria-induced epithelial-mesenchymal transition by regulating the PI3K-Akt-GSK-3 beta signaling pathway. Biochemical and Biophysical Research Communications, 463, 70–75. https://doi.org/10.1016/j.bbrc.2015.05.020.
Luo, Y., Wang, L., Peng, A., & Liu, J. Y. (2018). Metabolic profiling of human plasma reveals the activation of 5-lipoxygenase in the acute attack of gouty arthritis. Rheumatology.
Maccarrone, M., Meloni, C., Manca-di-Villahermosa, S., Cococcetta, N., Casciani, C. U., Finazzi-Agro, A., et al. (2001). Vitamin E suppresses 5-lipoxygenase-mediated oxidative stress in peripheral blood mononuclear cells of hemodialysis patients regardless of administration route. American Journal of Kidney Diseases, 37, 964–969. https://doi.org/10.1053/ajkd.2001.23617.
MacIsaac, R. J., Ekinci, E. I., Premaratne, E., Lu, Z. X., Seah, J. M., Li, Y., et al. (2015). The chronic kidney disease-epidemiology collaboration (CKD-EPI) equation does not improve the underestimation of glomerular filtration rate (GFR) in people with diabetes and preserved renal function. BMC Nephrology, 16, 198. https://doi.org/10.1186/s12882-015-0196-0.
Matsunaga, T., Shintani, S., & Hara, A. (2006). Multiplicity of mammalian reductases for xenobiotic carbonyl compounds. Drug Metabolism and Pharmacokinetics, 21, 1–18.
Meyer, T. W., & Hostetter, T. H. (2007). Uremia. The New England Journal of Medicine, 357, 1316–1325. https://doi.org/10.1056/NEJMra071313.
Munoz, M., Sanchez, A., Martinez, M. P., Benedito, S., Lopez-Oliva, M. E., Garcia-Sacristan, A., et al. (2015). COX-2 is involved in vascular oxidative stress and endothelial dysfunction of renal interlobar arteries from obese Zucker rats. Free Radical Biology & Medicine, 84, 77–90. https://doi.org/10.1016/j.freeradbiomed.2015.03.024.
Nair, M., le Roux, C. W., & Docherty, N. G. (2016). Measuring changes in renal function after bariatric surgery: Why estimated glomerular filtration rate is not good enough. Surgery for Obesity and Related Diseases, 12, 1897–1898. https://doi.org/10.1016/j.soard.2016.03.027.
Nakanishi, M., Kakumoto, M., Matsuura, K., Deyashiki, Y., Tanaka, N., Nonaka, T., et al. (1996). Involvement of two basic residues (Lys-17 and Arg-39) of mouse lung carbonyl reductase in NADP(H)-binding and fatty acid activation: Site-directed mutagenesis and kinetic analyses. The Journal of Biochemistry, 120, 257–263.
Nentwig, A., Schweighauser, A., Maissen-Villiger, C., Bruckmaier, R. M., Zurbriggen, A., van Dorland, H. A., et al. (2016). Assessment of the expression of biomarkers of uremic inflammation in dogs with renal disease. American Journal of Veterinary Research, 77, 218–224. https://doi.org/10.2460/ajvr.77.2.218.
Nolin, T. D. (2017). Drug metabolism in kidney disease. Drug Metabolism in Diseases. https://doi.org/10.1016/B978-0-12-802949-7.00004-3.
Norris, P. C., & Dennis, E. A. (2014). A lipidomic perspective on inflammatory macrophage eicosanoid signaling. Advances in Biological Regulation, 54, 99–110. https://doi.org/10.1016/j.jbior.2013.09.009.
Olearczyk, J. J., Quigley, J. E., Mitchell, B. C., Yamamoto, T., Kim, I. H., Newman, J. W., et al. (2009). Administration of a substituted adamantyl urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clinical Science (London), 116, 61–70. https://doi.org/10.1042/CS20080039.
Peiris, H. N., Vaswani, K., Almughlliq, F., Koh, Y. Q., & Mitchell, M. D. (2017). Review: Eicosanoids in preterm labor and delivery: Potential roles of exosomes in eicosanoid functions. Placenta, 54, 95–103. https://doi.org/10.1016/j.placenta.2016.12.013.
Peters, V., Klessens, C. Q., Baelde, H. J., Singler, B., Veraar, K. A., Zutinic, A., et al. (2015). Intrinsic carnosine metabolism in the human kidney. Amino Acids, 47, 2541–2550. https://doi.org/10.1007/s00726-015-2045-7.
Richardson, J. (2006). How to measure renal function in clinical practice: Estimated glomerular filtration rate in general practice. BMJ, 333, 918. https://doi.org/10.1136/bmj.333.7574.918-a.
Rink, N., & Zappitelli, M. (2015). Estimation of glomerular filtration rate with and without height: Effect of age and renal function level. Pediatric Nephrology, 30, 1327–1336. https://doi.org/10.1007/s00467-015-3063-0.
Robinson, R., Tait, C. D., Somov, P., Lau, M. W., Sangar, V. K., Ramani, V. A., et al. (2016). Estimated glomerular filtration rate is unreliable in detecting renal function loss during follow-up after cystectomy and urinary diversion. International Urology and Nephrology, 48, 511–515. https://doi.org/10.1007/s11255-016-1216-0.
Roche, C., Guerrot, D., Harouki, N., Duflot, T., Besnier, M., Remy-Jouet, I., et al. (2015). Impact of soluble epoxide hydrolase inhibition on early kidney damage in hyperglycemic overweight mice. Prostaglandins & Other Lipid Mediators, 120, 148–154. https://doi.org/10.1016/j.prostaglandins.2015.04.011.
Sahu, P. K., Pal, A., Panda, J., & Patnaik, S. (2011). Diuretics induced uremia and nonrecovery of renal function in a patient with acute renal failure caused by sepsis. Indian Journal of Pharmacology, 43, 603–604. https://doi.org/10.4103/0253-7613.84983.
Sanak, M. (2016). Eicosanoid mediators in the airway inflammation of asthmatic patients: What is new? Allergy, Asthma & Immunology Research: AAIR, 8, 481–490. https://doi.org/10.4168/aair.2016.8.6.481.
Schwartzman, M. L., Martasek, P., Rios, A. R., Levere, R. D., Solangi, K., Goodman, A. I., et al. (1990). Cytochrome P450-dependent arachidonic acid metabolism in human kidney. Kidney International, 37, 94–99.
Smith, H. E., Jones, J. P., Kalhorn, T. F., Farin, F. M., Stapleton, P. L., Davis, C. L., et al. (2008). Role of cytochrome P450 2C8 and 2J2 genotypes in calcineurin inhibitor-induced chronic kidney disease. Pharmacogenetics and Genomics, 18, 943–953. https://doi.org/10.1097/FPC.0b013e32830e1e16.
Stevens, L. A., Coresh, J., Greene, T., & Levey, A. S. (2006). Assessing kidney function—measured and estimated glomerular filtration rate. The New England Journal of Medicine, 354, 2473–2483. https://doi.org/10.1056/NEJMra054415.
Tinguely, J. N., & Wermuth, B. (1999). Identification of the reactive cysteine residue (Cys227) in human carbonyl reductase. European Journal of Biochemistry, 260, 9–14.
Tuncer, S., & Banerjee, S. (2015). Eicosanoid pathway in colorectal cancer: Recent updates. World Journal of Gastroenterology, 21, 11748–11766. https://doi.org/10.3748/wjg.v21.i41.11748.
Velenosi, T. J., Fu, A. Y., Luo, S., Wang, H., & Urquhart, B. L. (2012). Down-regulation of hepatic CYP3A and CYP2C mediated metabolism in rats with moderate chronic kidney disease. Drug Metabolism & Disposition, 40, 1508–1514. https://doi.org/10.1124/dmd.112.045245.
Violi, F., Targher, G., Vestri, A., Carnevale, R., Averna, M., Farcomeni, A., et al. (2017). Effect of aspirin on renal disease progression in patients with type 2 diabetes: A multicenter, double-blind, placebo-controlled, randomized trial. The renaL disEase progression by aspirin in diabetic pAtients (LEDA) trial. Rationale and study design. American Heart Journal, 189, 120–127. https://doi.org/10.1016/j.ahj.2017.04.005.
Vona-Davis, L., & Rose, D. P. (2013). The obesity-inflammation-eicosanoid axis in breast cancer. Journal of Mammary Gland Biology and Neoplasia, 18, 291–307. https://doi.org/10.1007/s10911-013-9299-z.
Wang, Q., Pang, W., Cui, Z., Shi, J. B., Liu, Y., Liu, B., et al. (2013). Upregulation of soluble epoxide hydrolase in proximal tubular cells mediated proteinuria-induced renal damage. American Journal of Physiology-Renal Physiology, 304, F168–F176. https://doi.org/10.1152/ajprenal.00129.2012.
Yu, Z., Davis, B. B., Morisseau, C., Hammock, B. D., Olson, J. L., Kroetz, D. L., et al. (2004). Vascular localization of soluble epoxide hydrolase in the human kidney. Journal of the American Society of Nephrology, 286, F720–F726. https://doi.org/10.1152/ajprenal.00165.2003.
Zhang, Q. A., Qiu, J. S., Li, H. M., Lu, Y. W., Wang, X. Y., Yang, J. W., et al. (2011). Cyclooxygenase 2 promotes parathyroid hyperplasia in ESRD. Journal of the American Society of Nephrology, 22, 664–672. https://doi.org/10.1681/Asn.2010060594.
Zhao, X., Yamamoto, T., Newman, J. W., Kim, I. H., Watanabe, T., Hammock, B. D., et al. (2004). Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. Journal of the American Society of Nephrology, 15, 1244–1253.
Zhou, S., & Glowacki, J. (2017). Chronic kidney disease and vitamin D metabolism in human bone marrow-derived MSCs. Annals of the New York Academy of Sciences, 1402, 43–55. https://doi.org/10.1111/nyas.13464.
Zivkovic, A. M., Yang, J., Georgi, K., Hegedus, C., Nording, M. L., O’Sullivan, A., et al. (2012). Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics, 8, 1102–1113. https://doi.org/10.1007/s11306-012-0417-5.
Acknowledgements
This study was co-supported by Shanghai Science and Technology Committee (STCSM) Grant 17511110205 (D.-Y.H.), National Natural Science Foundation of China (NSFC) Grant 81470588 (J.-Y.L.), Fundamental Research Funds for the Central Universities Grant 2016KJ048 (Y.L.), and the Tenth People’s Hospital of Tongji University 3.50 Clinical Project DS04.99.17013 (D.-Y.H.). The authors would like to thank all the uremic patients and healthy volunteers for the participation in this study.
Author information
Authors and Affiliations
Contributions
J-YL designed and supervised the study. D-YH, YL, and J-YL conducted sample analysis and data analysis. D-YH, C-BL, X-HL, and AP performed clinical diagnosis. D-YH, YL, C-BL, and C-YZ collected samples and clinic information. J-YL wrote the paper. All the authors critically reviewed the paper and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments and approved by the Ethics Committee of Shanghai Tenth People’s Hospital.
Informed consent
Informed consent was obtained from all individual participants in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, DY., Luo, Y., Li, CB. et al. Oxylipin profiling of human plasma reflects the renal dysfunction in uremic patients. Metabolomics 14, 104 (2018). https://doi.org/10.1007/s11306-018-1402-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-018-1402-4