Abstract
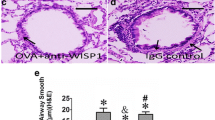
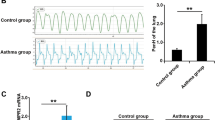
The P2X4 receptor (P2X4R) contributes to airway inflammation and airway remodeling in mice with allergic asthma. However, the molecular mechanism by which P2X4R affects the airway remodeling in allergic asthma remains largely unknown. We established an allergic asthma model by ovalbumin (OVA) inhalation in BALB/c mice. Compared with the mice in the control group, the expression of proliferating cell nuclear antigen (PCNA) increased and that of alpha-smooth muscle actin (α-SMA) decreased in the OVA-challenged mice. 5-BDBD, a P2X4R antagonist, alleviated the OVA-induced changes. To clarify the role of P2X4R in the phenotype switching of the bronchial smooth muscle, bronchial smooth muscle contractility and p38MAPK expression were investigated. Platelet-derived growth factor-BB (PDGF-BB) was used to activate the proliferation of primary-cultured rat bronchial smooth muscle cells (BSMCs). P2X4R, p38MAPK, and phenotype markers were evaluated using Western blotting or immunofluorescence. PDGF-BB administration increased the P2X4R and phospho-p38MAPK expression in BSMCs, and the increased phospho-p38MAPK expression was downregulated by silencing of the P2X4R mRNA. PDGF-BB stimulated the proliferation and synthetic phenotype of BSMCs, which was aggravated by a P2X4R agonist and alleviated by a P2X4R antagonist or silencing the P2X4R mRNA. The decreased contractile phenotype induced by PDGF-BB was alleviated by a P2X4R antagonist or by silencing the P2X4R mRNA. SB203580, p38MAPK inhibitor, inhibited the PDGF-BB-induced increasing of synthetic phenotype and the proliferation of BSMCs. These findings indicate that P2X4R acts directly on the phenotype switching of BSMCs. Inhibiting P2X4R can promote the contractile differentiation of BSMCs via p38MAPK signaling. Thus, the effect of P2X4R on airway remodeling indicates that this receptor could be a target for future drug candidates.







Similar content being viewed by others
References
Lauzon AM, Martin JG (2016) Airway hyperresponsiveness; smooth muscle as the principal actor. F1000Res 5. https://doi.org/10.12688/f1000research.7422.1
Chiba Y, Tanoue G, Suto R, Suto W, Hanazaki M, Katayama H, Sakai H (2017) Interleukin-17a directly acts on bronchial smooth muscle cells and augments the contractility. Pharmacol Rep 69:377–385. https://doi.org/10.1016/j.pharep.2016.12.007
Girodet PO, Allard B, Thumerel M, Begueret H, Dupin I, Ousova O, Lassalle R, Maurat E, Ozier A, Trian T, Marthan R, Berger P (2016) Bronchial smooth muscle remodeling in nonsevere asthma. Am J Respir Crit Care Med 193:627–633. https://doi.org/10.1164/rccm.201507-1404OC
Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M (2003) Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 167:1360–1368. https://doi.org/10.1164/rccm.200209-1030OC
Yu ZH, Wang YX, Song Y, Lu HZ, Hou LN, Cui YY, Chen HZ (2013) Up-regulation of kca3.1 promotes human airway smooth muscle cell phenotypic modulation. Pharmacol Res 77:30–38. https://doi.org/10.1016/j.phrs.2013.09.002
Halayko AJ, Stephens NL (1994) Potential role for phenotypic modulation of bronchial smooth muscle cells in chronic asthma. Can J Physiol Pharmacol 72:1448–1457
Movassagh H, Tatari N, Shan L, Koussih L, Alsubait D, Khattabi M, Redhu NS, Roth M, Tamm M, Chakir J, Gounni AS (2016) Human airway smooth muscle cell proliferation from asthmatics is negatively regulated by semaphorin3a. Oncotarget 7:80238–80251. https://doi.org/10.18632/oncotarget.12884
Jin H, Wang Y, Zhou L, Liu L, Zhang P, Deng W, Yuan Y (2014) Melatonin attenuates hypoxic pulmonary hypertension by inhibiting the inflammation and the proliferation of pulmonary arterial smooth muscle cells. J Pineal Res 57:442–450. https://doi.org/10.1111/jpi.12184
Hall PA, Levison DA, Woods AL, Yu CC, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R et al (1990) Proliferating cell nuclear antigen (pcna) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol 162:285–294. https://doi.org/10.1002/path.1711620403
Stumm CL, Halcsik E, Landgraf RG, Camara NO, Sogayar MC, Jancar S (2014) Lung remodeling in a mouse model of asthma involves a balance between tgf-beta1 and bmp-7. PLoS One 9:e95959. https://doi.org/10.1371/journal.pone.0095959
Zhao L, Wu J, Zhang X, Kuang H, Guo Y, Ma L (2013) The effect of Shenmai injection on the proliferation of rat airway smooth muscle cells in asthma and underlying mechanism. BMC Complement Altern Med 13:221. https://doi.org/10.1186/1472-6882-13-221
Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC (2006) Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 112:358–404. https://doi.org/10.1016/j.pharmthera.2005.04.013
Eltzschig HK, Sitkovsky MV, Robson SC (2013) Purinergic signaling during inflammation. N Engl J Med 368:1260–1261. https://doi.org/10.1056/NEJMc1300259
Idzko M, Ferrari D, Eltzschig HK (2014) Nucleotide signalling during inflammation. Nature 509:310–317. https://doi.org/10.1038/nature13085
Zech A, Wiesler B, Ayata CK, Schlaich T, Durk T, Hossfeld M, Ehrat N, Cicko S, Idzko M (2016) P2rx4 deficiency in mice alleviates allergen-induced airway inflammation. Oncotarget 7:80288–80297. https://doi.org/10.18632/oncotarget.13375
Kesavan R, Potunuru UR, Nastasijevic B, T A, Joksic G, Dixit M (2013) Inhibition of vascular smooth muscle cell proliferation by Gentiana lutea root extracts. PLoS One 8:e61393. https://doi.org/10.1371/journal.pone.0061393
Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, Couillin I (2010) Extracellular atp is a danger signal activating p2x7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182:774–783. https://doi.org/10.1164/rccm.201003-0359OC
Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K (2009) Behavioral phenotypes of mice lacking purinergic p2x4 receptors in acute and chronic pain assays. Mol Pain 5:28. https://doi.org/10.1186/1744-8069-5-28
Vanacker NJ, Palmans E, Kips JC, Pauwels RA (2001) Fluticasone inhibits but does not reverse allergen-induced structural airway changes. Am J Respir Crit Care Med 163:674–679
Chen H, Xia Q, Feng X, Cao F, Yu H, Song Y, Ni X (2016) Effect of p2x4r on airway inflammation and airway remodeling in allergic airway challenge in mice. Mol Med Rep 13:697–704. https://doi.org/10.3892/mmr.2015.4622
Ni X, Li X, Fang X, Li N, Cui W, Zhang B (2010) Ngf/trka-mediated kidins220/arms signaling activated in the allergic airway challenge in mice. Ann Allergy Asthma Immunol 105:299–306. https://doi.org/10.1016/j.anai.2010.08.006
Dai YR, Wu HY, Wu LQ, Xu H, Yin J, Yan SS, Zeng WX (2014) Roxithromycin reduces the viability of cultured airway smooth muscle cells from a rat model of asthma. Eur Rev Med Pharmacol Sci 18:3564–3572
Qiu C, Zhang J, Su M, Fan X (2015) Nuclear factor-kappab mediates the phenotype switching of airway smooth muscle cells in a murine asthma model. Int J Clin Exp Pathol 8:12115–12128
Balazs B, Danko T, Kovacs G, Koles L, Hediger MA, Zsembery A (2013) Investigation of the inhibitory effects of the benzodiazepine derivative, 5-bdbd on p2x4 purinergic receptors by two complementary methods. Cell Physiol Biochem 32:11–24. https://doi.org/10.1159/000350119
Kwon HJ (2012) Extracellular atp signaling via p2x(4) receptor and camp/pka signaling mediate atp oscillations essential for prechondrogenic condensation. J Endocrinol 214:337–348. https://doi.org/10.1530/JOE-12-0131
Bergeron C, Tulic MK, Hamid Q (2010) Airway remodelling in asthma: from benchside to clinical practice. Can Respir J 17:e85–e93
Brightling CE, Gupta S, Gonem S, Siddiqui S (2012) Lung damage and airway remodelling in severe asthma. Clin Exp Allergy 42:638–649. https://doi.org/10.1111/j.1365-2222.2011.03917.x
Hartley RA, Barker BL, Newby C, Pakkal M, Baldi S, Kajekar R, Kay R, Laurencin M, Marshall RP, Sousa AR, Parmar H, Siddiqui S, Gupta S, Brightling CE (2016) Relationship between lung function and quantitative computed tomographic parameters of airway remodeling, air trapping, and emphysema in patients with asthma and chronic obstructive pulmonary disease: a single-center study. J Allergy Clin Immunol 137:1413–1422 e1412. https://doi.org/10.1016/j.jaci.2016.02.001
O'Reilly R, Ullmann N, Irving S, Bossley CJ, Sonnappa S, Zhu J, Oates T, Banya W, Jeffery PK, Bush A, Saglani S (2013) Increased airway smooth muscle in preschool wheezers who have asthma at school age. J Allergy Clin Immunol 131:1024–1032, 1032 e1021-1016. https://doi.org/10.1016/j.jaci.2012.08.044
Hirota N, Martin JG (2013) Mechanisms of airway remodeling. Chest 144:1026–1032. https://doi.org/10.1378/chest.12-3073
Sun Q, Liu L, Wang H, Mandal J, Khan P, Hostettler KE, Stolz D, Tamm M, Molino A, Lardinois D, Lu S, Roth M (2017) Constitutive high expression of protein arginine methyltransferase 1 in asthmatic airway smooth muscle cells is caused by reduced microrna-19a expression and leads to enhanced remodeling. J Allergy Clin Immunol 140:510–524 e513. https://doi.org/10.1016/j.jaci.2016.11.013
Hassan M, Jo T, Risse PA, Tolloczko B, Lemiere C, Olivenstein R, Hamid Q, Martin JG (2010) Airway smooth muscle remodeling is a dynamic process in severe long-standing asthma. J Allergy Clin Immunol 125:1037–1045 e1033. https://doi.org/10.1016/j.jaci.2010.02.031
Papi A, Brightling C, Pedersen SE, Reddel HK (2018) Asthma. Lancet 391:783–800. https://doi.org/10.1016/S0140-6736(17)33311-1
Wareham K, Vial C, Wykes RC, Bradding P, Seward EP (2009) Functional evidence for the expression of p2x1, p2x4 and p2x7 receptors in human lung mast cells. Br J Pharmacol 157:1215–1224. https://doi.org/10.1111/j.1476-5381.2009.00287.x
Wu T, Dai M, Shi XR, Jiang ZG, Nuttall AL (2011) Functional expression of p2x4 receptor in capillary endothelial cells of the cochlear spiral ligament and its role in regulating the capillary diameter. Am J Physiol Heart Circ Physiol 301:H69–H78. https://doi.org/10.1152/ajpheart.01035.2010
Harhun MI, Povstyan OV, Albert AP, Nichols CM (2014) Atp-evoked sustained vasoconstrictions mediated by heteromeric p2x1/4 receptors in cerebral arteries. Stroke 45:2444–2450. https://doi.org/10.1161/STROKEAHA.114.005544
Ravanti L, Toriseva M, Penttinen R, Crombleholme T, Foschi M, Han J, Kahari VM (2001) Expression of human collagenase-3 (mmp-13) by fetal skin fibroblasts is induced by transforming growth factor beta via p38 mitogen-activated protein kinase. FASEB J 15:1098–1100
Stellato C, Brummet ME, Plitt JR, Shahabuddin S, Baroody FM, Liu MC, Ponath PD, Beck LA (2001) Expression of the c-c chemokine receptor ccr3 in human airway epithelial cells. J Immunol 166:1457–1461
Jin XH, Wang LN, Zuo JL, Yang JP, Liu SL (2014) P2x4 receptor in the dorsal horn partially contributes to brain-derived neurotrophic factor oversecretion and toll-like receptor-4 receptor activation associated with bone cancer pain. J Neurosci Res 92:1690–1702. https://doi.org/10.1002/jnr.23443
Williams AS, Issa R, Durham A, Leung SY, Kapoun A, Medicherla S, Higgins LS, Adcock IM, Chung KF (2008) Role of p38 mitogen-activated protein kinase in ozone-induced airway hyperresponsiveness and inflammation. Eur J Pharmacol 600:117–122. https://doi.org/10.1016/j.ejphar.2008.09.031
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81200011) and the Natural Science Foundation of Heilongjiang Province (grant number H2016021) and Harbin Medical University-Daqing Seed Found Project (grant number DQXN201608).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests. The authors alone are responsible for the content and writing of this paper.
Ethical approval
The experimental protocols were approved by the Animal Care and Protection Committee of Harbin Medical University-Daqing. Our use of animals conformed to our Institution’s and country’s animal welfare laws, and our studies were approved.
Rights and permissions
About this article
Cite this article
Wang, L., Feng, X., Hu, B. et al. P2X4R promotes airway remodeling by acting on the phenotype switching of bronchial smooth muscle cells in rats. Purinergic Signalling 14, 433–442 (2018). https://doi.org/10.1007/s11302-018-9625-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-018-9625-4