Abstract
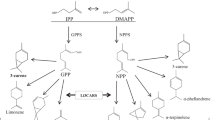
Mountain pepper (Litsea cubeba (Lour.) Persoon) is an evergreen tree in the Lauraceae family and has attracted attention for the antioxidant and antibacterial effects of its essential oils. The dominant components of the essential oil of L. cubeba are monoterpenes. To investigate the biosynthesis of monoterpenes that have bioactivity in L. cubeba, three cDNAs corresponding to monoterpene synthase genes were isolated, and the corresponding enzymes were functionally characterized in vitro. The genes obtained were named LcTPS1, LcTPS2, and LcTPS3. Transcript levels for LcTPS1 were most prominent in the leaf tissues, and levels of LcTPS2 and LcTPS3 were more evident in fruit tissues than in leaf tissues. Phylogenetic analyses indicated that LcTPS1, LcTPS2, and LcTPS3 all belong to TPS-b subfamily of angiosperm monoterpene synthases. For each genes, the corresponding enzymes were expressed and functionally characterized. LcTPS1 converted geranyl diphosphate to trans-ocimene, and LcTPS2 converted geranyl diphosphate to α-thujene, while LcTPS3 is a mutifunctional enzyme that converted geranyl diphosphate to α-thujene and (+)-sabinene, respectively.






Similar content being viewed by others
Abbreviations
- mono-TPS:
-
Monoterpene synthase
- TPS:
-
Terpene synthase
References
Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J (2000) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406:512–515
Bergstrom G, Dobson HEM, Groth I (1995) Spatial fragrance patterns within the flowers of Ranunculus acris (Ranunculaceae). Plant Syst Evol 195:221–242
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci 95:4126–4133
Bohlmann J, Martin D, Oldham NJ, Gershenzon J (2000) Terpenoid secondary metabolism in Arabidopsis thaliana: cDNA cloning, characterization, and functional expression of a myrcene/(E)-β-ocimene synthase. Arch Biochem Biophys 375:261–269
Borg-Karlson AK, Englund FO, Unelius CR (1994) Dimethyl oligosulphides, major volatiles released from Sauromatum gottatum and Phallus impudicus. Phytochemistry 35:321–323
Buckingham J (1998) Dictionary of natural products on CD-ROM, version 6.1. Chapman and Hall, London
Chen F, Tholl D, D’Auria JC, Farooq A, Pichersky E, Gershenzon J (2003) Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 15:481–494
Chen F, Ro DK, Petri J, Gershenzon J, Bohlmann J, Pichersky E, Tholl D (2004) Characterization of a root-specific Arabidopsis terpene synthase responsible for the formation of the volatile monoterpene 1,8-cineole1. Plant Physiol 135:1956–1966
Christianson DW (2006) Structural biology and chemistry of the terpenoid cyclases. Chem Rev 106:3412–3442
Coudhunry S, Ahmed R, Barthel A, Leclercq PA (1998) Composition of the stem, flower and fruit oils of Litsea cubeba Pers. From two locations of Assam, India. J Essent Oil Res 10:381–386
Cox B, Kislinger T, Emili A (2004) Integrating gene and protein expression data: pattern analysis and profile mining. Methods 35:303–314
Croteau R (1987) Biosynthesis and catabolism of monoterpenoids. Chem Rev 87:929–954
Davis EM, Croteau R (2000) Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. Top Curr Chem 209:53–95
Degenhardt J, Köllner TG, Gershenzon J (2009) Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70:1621–1637
Duke JA, Ayensu ES (1985) Medicinal plants of China. Reference Publications, Michigan, pp 390–391
Elzen GW, Williams HJ, Bell AA, Stipanovic RD, Vinson SB (1985) Quantification of volatile terpenes of glanded and glandless Gossypium hirsutum L. cultivars and lines by gas chromatography. J Agric Food Chem 33:1079–1082
Fabbro SD, Nazzi F (2008) Repellent effect of sweet basil compounds on Ixodes ricinus ticks. Exp Appl Acarol 45:219–228
Fäldt J, Martin D, Miller B, Rawat S, Bohlmann J (2003) Traumatic resin defense in Norway spruce (Picea abies): methyl jasmonate-induced terpene synthase gene expression, and cDNA cloning and functional characterization of (+)-3-carene synthase. Plant Mol Biol 51:119–133
Ho CL, Jie-Pinge O, Liu YC, Hung CP, Tsai MC, Liao PC, Wang EI, Chen YL, Su YC (2010) Compositions and in vitro anticancer activities of the leaf and fruit oils of Litsea cubeba from Taiwan. Nat Prod Commun 5:617–620
Hwang JK, Choi EM, Lee JH (2005) Antioxidant activity of Litsea cubeba. Fitoterapia 76:684–686
Hyatt DC, Youn B, Zhao Y, Santhamma B, Coates RM, Croteau RB, Kang CH (2007) Structure of limonene synthase, a simple model for terpenoid cyclase catalysis. Proc Natl Acad Sci USA 104:5360–5365
Kampranis SC, Ioannidis D, Purvis A, Mahrez W, Ninga E, Katerelos NA, Anssour S, Dunwell JM, Degenhardt J, Makris AM, Goodenough PW, Johnson CB (2007) Rational conversion of substrate and product specificity in a salvia monoterpene synthase: structural insights into the evolution of terpene synthase function. Plant Cell 19:1994–2005
Kessler A, Baldwin IT (2010) Defensive function of herbivore-induced plant volatile emissions in nature. Sci 291:2141–2144
Kohzaki K, Gomi K, Kokudo YY, Ozawa R, Takabayashi J, Akimitsu K (2009) Characterization of a sabinene synthase gene from rough lemon (Citrus jambhiri). J Plant Physiol 166:1700–1704
Lagalante AF, Montgomery ME (2003) Analysis of terpenoids from Hemlock (Tsuga) species by solid-phase microextraction/gas chromatography/ion–trap mass spectrometry. J Agric Food Chem 51:2115–2120
Liu ZL, Goh SH, Ho SH (2007) Screening of Chinese medicinal herbs for bioactivity against Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J Stored Prod Res 43:290–296
Lücker J, Bowen P, Bohlmann J (2004) Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (+)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 65:2649–2659
Marriacci L, Dicke M, Posthumus MA (1994) Induction of parasitoid attracting synomone in brussels sprouts plants by feeding of Pieris brassicae larvae: role of mechanical damage and herbivore elicitor. J Chem Ecol 20:2229–2247
Martin DM, Faldt J, Bohlmann J (2004) Functional characterization of nine Norway spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant Physiol 135:1908–1927
Page RDM (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Schnee C, Kollner TG, Gershenzon J, Degenhardt J (2002) The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-beta-farnesene, (E)-nerolidol, and (E, E)-farnesol after herbivore damage. Plant Physiol 130:2049–2052
Seemann M, Zhai GZ, de Kraker JW, Paschall CM, Christianson DW, Cane DE (2002) Pentalenene synthase. Analysis of active site residues by site-directed mutagenesis. J Am Chem Soc 124:7681–7689
Starks CM, Back K, Chappell J, Noel JP (1997) Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Sci 277:1815–1820
Tholl D (2006) Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol 9:297–304
Trapp SC, Croteau RB (2001) Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 158:811–832
Wang H, Liu Y (2010) Chemical composition and antibacterial activity of essential oils from different parts of Litsea cubeba. Chem Biodiv 7:229–235
Whittington DA, Wise ML, Urbansky M, Coates RM, Croteau RB, Christianson DW (2002) Bornyl diphosphate synthase: structure and strategy for carbocation manipulation by a terpenoid cyclase. Natl Acad Sci USA 99:15375–15380
Wise ML, Croteau R (1999) Comprehensive natural products chemistry: isoprenoids including caroteinoids and steroids. Sci 2:97–135
Acknowledgments
The authors are grateful for expert technical assistance from Yi-Ru Lee and the Huisun Experimental Forest of the National Chung Hsing University for providing various organ samples of L. cubeba. Financial assistance from the Council of Agriculture Executive Yuan is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Sederoff
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Figure 1
Homology models for LcTPS1. The models were constructed using SWISS protein structure prediction server by threading LcTPS1 sequences into the coordinate of (4S)-limonene synthase (PDB code: 2ongB) three-dimensional structure. a Homology model for LcTPS1 that colored by secondary structure succession from N-terminal to C-terminal domain. b Homology model for LcTPS1 which the DDxxD motif (green), the NSE/DTE motif (blue), and the H-α1 loop region (purple) with their associated bound magnesium atoms (white tri-atom cluster) and the diphosphate moiety of 2-fluoro GPP (analogous of GPP) which is shown in sticks are also indicated. (DOC 513 kb)
Figure 2
Amino acids alignment showing the topological positioning of the H-α1 loop in nine TPS-d enzymes and the three TPS-b enzymes from L. cubeba. The nine TPS-d sequences were selected on the basis that they account for all possible variations of amino acids in the H-α1 loop of 24 TPS-d protein sequences. Aa linalool, Artemisia annua linalool synthase (AAF13356); Aa pinene, Artemisia annua ß -pinene synthase (AAK58723); At myrcene, Arabidopsis thaliana putative limonene cyclase (AAD03382); At limonene, Arabidopsis thalian limonene cyclase (BAB01181); Cu pinene, Citrus unshiu ß-pinene synthase (BAD27260); La bergamotene, Lavandula angustifolia trans-α-bergamotene synthase (ABB73046); La limonene, Lavandula angustifolia limonene synthase (ABB73044); Ma linalool Mentha aquati linalool synthase (AAL99381); Ob myrcene, Ocimum basilicum ß-myrcene synthase (AAV63791); Pml isoprene, Pueraria montana var. lobata isoprene synthase (AAQ84170); Pn isoprene, Populus nigra isoprene synthase (CAL69918); Qi myrcene, Quercus ilex putative chloroplast terpene synthase (CAC41012); Sf cineole Salvia fruticosa cineole synthase (ABH07677); So bornyl, Salvia officinalis (+)-bornyl diphosphate synthase (AAC26017). (DOC 320 kb)
Rights and permissions
About this article
Cite this article
Chang, YT., Chu, FH. Molecular cloning and characterization of monoterpene synthases from Litsea cubeba (Lour.) Persoon. Tree Genetics & Genomes 7, 835–844 (2011). https://doi.org/10.1007/s11295-011-0377-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-011-0377-3