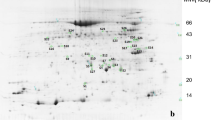
Abstract
In the temperate climate of the northern hemisphere, winter survival of woody plants is determined by the ability to acclimate to freezing temperatures and to undergo a period of dormancy. Cold acclimation in many woody plants is initially induced by short photoperiod and low, non-freezing temperatures. These two factors (5°C and short photoperiod) were used to study changes in the proteome of bark tissues of 1-year-old peach trees. Difference in-gel electrophoresis technology, a gel-based approach involving the labeling of proteins with different fluorescent dyes, was used to conduct a quantitative assessment of changes in the peach bark proteome during cold acclimation. Using this approach, we were able to identify differentially expressed proteins and to assign them to a class of either ‘temperature-responsive’ or ‘photoperiod-responsive’ proteins. The most significant factor affecting the proteome appeared to be low temperature, while the combination of low temperature and short photoperiod was shown to act either synergistically or additively on the expression of some proteins. Fifty-seven protein spots on gels were identified by mass spectrometry. They included proteins involved in carbohydrate metabolism (e.g., enolase, malate dehydrogenase, etc), defense or protective mechanisms (e.g., dehydrin, HSPs, and PR-proteins), energy production and electron transport (e.g., adenosine triphosphate synthases and lyases), and cytoskeleton organization (e.g., tubulins and actins). The information derived from the analysis of the proteome is discussed as a function of the two treatment factors: low temperature and short photoperiod.



Similar content being viewed by others
Abbreviations
- 2D (E):
-
bidimensional (electrophoresis)
- ABA:
-
abscisic acid
- CHAPS:
-
3-([3-cholamidopropyl]dimethylammonio)-1-propanesulfonate
- DiGE:
-
difference in-gel electrophoresis
- DTT:
-
dithiothreitol
- HSP:
-
heat-shock protein
- IPG:
-
immobilized pH gradient
- LD:
-
long day
- LT:
-
low temperature
- PR-proteins:
-
pathogenesis-related proteins
- SD:
-
short day
- TCA:
-
trichloroacetic acid
- SSH:
-
suppression subtractive hybridization
References
Antikainen M, Griffith M (1997) Antifreeze protein accumulation in freezing-tolerant cereals. Physiol Plant 99:423–432
Arora R, Wisniewski M (2000) Structural and biochemical aspects of cold hardiness in woody plants. In: Jain M, Minocha S (eds) Molecular biology of woody plants, vol. 2. Kluwer, Dordrecht, pp 419–438
Arora R, Wisniewski ME, Scorza R (1992) Cold acclimation in genetically related (sibling) deciduous and evergreen peach (Prunus persica [L.] Batsch). I Seasonal changes in cold hardiness and polypeptides of bark and xylem tissues. Plant Physiol 99:1562–1568
Artlip T, Wisniewski M (2002) Induction of proteins in response to biotic and abiotic stresses. In: Pessarakli M (ed) Handbook of plant and crop physiology. Marcell Dekker, New York, pp 657–680
Artlip TS, Callahan AM, Bassett CL, Wisniewski ME (1997) Seasonal expression of a dehydrin gene in sibling deciduous and evergreen genotypes of peach (Prunus persica [L] Batsch). Plant Mol Biol 33:61–70
Bae MN, Cho EJ, Choi EY, Park OK (2003) Analysis of the Arabidopsis nuclear proteome and its response to cold stress. Plant J 36:652–663
Bais HP, Vepachedu R, Lawrence CB, Stermitz FR, Vivanco JM (2003) Molecular and biochemical characterization of an enzyme responsible for the formation of hypericin in St. John’s Wort (Hypericum perforatum L.). J Biol Chem 278:32413–32422
Basset G, Raymond P, Malek L, Brouquisse R (2002) Changes in the expression and the enzymic properties of the 20S Proteasome in sugar-starved maize roots. Evidence for an in vivo oxidation of the proteasome. Plant Physiol 128:1149–1162
Bassett CL, Wisniewski ME, Artlip TS, Norelli JL, Renaut J, Farrel RE Jr (2006) Global analysis of genes regulated by low temperature and photoperiod in peach bark. J Am Soc Hort Sci 131:551–563
Benedict C, Skinner JS, Meng R, Chang Y, Bhalero R, Finn C, Chen THH, Hurry V (2006) The role of the CBF-dependent signalling pathway in woody perennials. In: Chen THH, Uemura M, Fujikawa S (eds) Cold hardiness in plants: molecular genetics, cell biology and physiology. CABI Pub, Oxfordshire, UK, pp 167–180
Bonhomme M, Rageau R, Richard JP, Erez A, Gendraud M (1999) Influence of three contrasted climatic conditions on endodormant vegetative and floral peach buds: analyses of their intrinsic growth capacity and their potential sink strength compared with adjacent tissues. Scientia Hortic 80:157–171
Ciereszko I, Johansson H, Kleczkowski LA (2001) Sucrose and light regulation of a cold-inducible UDP-glucose pyrophosphorylase gene via a hexokinase-independent and abscisic acid-insensitive pathway in Arabidopsis. Biochem J 354:67–72
Close TJ (1997) Dehydrins: a commonalty in the response of plants to dehydration and low temperature. Physiol.Plant. 100:291–296
Cox SE, Stushnoff C (2001) Temperature-related shifts in soluble carbohydrate content during dormancy and cold acclimation in Populus tremuloides. Can J For Res 31:730–737
El Kayal W, Navarro M, Marque G, Keller G, Marque C, Teulieres C (2006) Expression profile of CBF-like transcriptional factor genes from Eucalyptus in response to cold. J Exp Bot 57:2455–2469
Ferullo J-M, Griffith M (2001) Mechanisms of cold acclimation. In: Basra AS (ed) Crop responses and adaptations to temperature stress. Food Products Press, NY, pp 109–150
Forsthoefel NR, Cushman MAF, Cushman JC (1995) Posttranscriptional and posttranslational control of enolase expression in the facultative crassulacean acid metabolism plant Mesembryanthemum crystallinum L. Plant Physiol 108:1185–1195
Fowler S, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14:1675–1690
Fujii K, Kondo T, Yokoo H, Yamada T, Matsuno Y, Iwatsuki K, Hirohashi S (2005) Protein expression pattern distinguishes different lymphoid neoplasms. Proteomics 5:4274–4286
Gygi SP, Rochon Y, Franza BR, Aebersold R (1999) Correlation between protein and mRNA abundance in yeast. Mol Cell Bio 19:1720–1730
Griffith M, Yaish MWF (2004) Antifreeze proteins in overwintering plants: a tale of two activities. Trends Plant Sci 9:399–405
Griffith M, Lumb C, Wiseman SB, Wisniewski M, Johnson RW, Marangoni AG (2005) Antifreeze proteins modify the freezing process in planta. Plant Physiol 138:330–340
Guo D, Chen F, Inoue K, Blount JW, Dixon RA (2001) Downregulation of caffeic acid 3-O-methyltransferase and caffeoyl CoA 3-O-methyltransferase in transgenic alfalfa: impacts on lignin structure and implications for the biosynthesis of G and S lignin. Plant Cell 13:73–88
Halliday KJ, Fankhauser C (2003) Phytochrome-hormonal signalling networks. New Phytol 157:449–463
Hausman JF, Evers D, Thiellement H, Jouve L (2000) Compared responses of poplar cuttings and in vitro raised shoots to short-term chilling treatments. Plant Cell Rep 19:954–960
Hincha DK, Meins F Jr, Schmitt JM (1997) Beta-1,3-glucanase is cryoprotective in vitro and is accumulated in leaves during cold acclimation. Plant Physiol 114:1077–1083
Huang T, Duman JG (2002) Cloning and characterization of a thermal hysteresis (antifreeze) protein with DNA-binding activity from winter bittersweet nightshade, Solanum dulcamara. Plant Mol Biol 48:339–350
Imin N, Kerim T, Rolfe BG, Weinman JJ (2004) Effect of early cold stress on the maturation of rice anthers. Protc 4:1873–1882
Jakab G, Ton J, Flors V, Zimmerli L, Metraux JP, Mauch-Mani B (2005) Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol 139:267–274
Kawamura Y, Uemura M (2003) Mass spectrometric approach for identifying putative plasma membrane proteins of Arabidopsis leaves associated with cold acclimation. Plant J 36:141–154
Kleczkowski LA, Geisler M, Ciereszko I, Johansson H (2004) UDP-glucose pyrophosphorylase. An old protein with new tricks. Plant Physiol 134:912–918
Kozlowski TT (2002) Acclimation and adaptive responses of woody plants to environmental stresses. Botanical Rev 68:270–334
Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, Harper JF (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol 130:2129
Kuwabara C, Takezawa D, Shimada T, Hamada T, Fujikawa S, Arakawa K (2002) Abscisic acid- and cold-induced thaumatin-like protein in winter wheat has an antifungal activity against snow mould, Microdochium nivale. Physiol Plant 115:101–110
Lim CC, Krebs SL, Arora R (1999) A 25-kDa dehydrin associated with genotype and age-dependent leaf freezing-tolerance in Rhododendron: a genetic marker for cold hardiness? Theor Appl Genet 99:912–920
Liu JJ, Ekramoddoullah AKM, Yu X (2003) Differential expression of multiple PR10 proteins in western white pine following wounding, fungal infection and cold-hardening. Physiol Plant 119:544–553
Liu JJ, Ekramoddoullah AKM, Taylor D, Piggott N, Lane S, Hawkins B (2004) Characterization of Picg5 novel proteins associated with seasonal cold acclimation of white spruce (Picea glauca). Trees 18:649–657
Marian CO, Krebs CL, Arora R (2004) Dehydrin variability among rhododendron species: a 25-kDa dehydrin is conserved and associated with cold acclimation across diverse species. New Phytol 161:773–780
Muthalif MM, Rowland LJ (1994) Identification of dehydrin-like proteins responsive to chilling in floral buds of blueberry (Vaccinium, section Cyanoccoccus). Plant Physiol 104:1439–1447
Neven LG, Haskell DW, Guy CL, Denslow N, Klein PA, Green LG, Silverman A (1992) Association of 70-kilodalton heat-shock cognate proteins with acclimation to cold. Plant Physiol 99:1362–1369
Pichler FB, Walton EF, Davy M, Triggs C, Janssen B, Wünsche JN, Putterill J, Schaffer RJ (2007) Relative developmental, environmental, and tree-to-tree variability in buds from field-grown apple trees. Tree Genetics & Genomes 3:329–339
Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol 133:1755–1767
Renaut J, Lutts S, Hoffmann L, Hausman JF (2004) Responses of poplar to chilling temperatures: proteomic and physiological aspects. Plant Biol 6:81–90
Renaut J, Hoffmann L, Hausman JF (2005) Biochemical and physiological mechanisms related to cold acclimation and enhanced freezing tolerance in poplar plantlets. Physiol Plant 125:82–94
Renaut J, Hausman JF, Wisniewski ME (2006) Proteomics and low-temperature studies: bridging the gap between gene expression and metabolism. Physiol Plant 126:97–109
Ribeiro A, Praekelt U, Akkermans ADL, Meacock PA, van Kammen A, Bisseling T, Pawlowski K (1996) Identification of agthi1, whose product is involved in biosynthesis of the thiamine precursor thiazole, in actinorhizal nodules of Alnus glutinosa. Plant J 10:361–368
Rinne PL, Kaikuranta PL, vanderPlas LH, vanderSchoot C (1999) Dehydrins in cold-acclimated apices of birch (Betula pubescens Ehrh.): production, localization and potential role in rescuing enzyme function during dehydration. Planta 209:377–388
Rose JKC, Bashir S, Giovannoni JJ, Jahn MM, Saravanan RS (2004) Tackling the plant proteome: practical approaches, hurdles and experimental tools. Plant J 39:715–733
Sakai A, Larcher W (1987) Frost survival of plants. Responses and adaptation to freezing stress. Springer, Berlin
Sauter JJ, van Cleve B (1994) Storage, mobilization and interrelations of starch, sugars, protein and fat in the ray storage tissue of poplar trees. Trees 8:297–304
Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K (2001) Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell 13:61–72
Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M, Akiyama K, Taji T, Yamaguchi-Shinozaki K, Carninci P, Kawai J, Hayashizaki Y, Shinozaki K (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31:279–292
Skynner HA, Rosahl TW, Knowles MR, Salim K, Reid L, Cothliff R, McAllister G, Guest PC (2002) Alterations of stress related proteins in genetically altered mice revealed by two-dimensional differential in-gel electrophoresis analysis. Proteomics 2:1018–1025
Sung DY, Vierling E, Guy CL (2001) Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol 126:789–800
Stushnoff C, Seufferheld MJ, Creegan T (1998) Oligosaccharides as endogenous cryoprotectants in woody plants. In: Li PH, Chen THH (eds) plant cold hardiness. Molecular biology, biochemistry and physiology. Plenum, New York, pp 301–309
Taylor NT, Heazlewood JL, Day DA, Millar AH (2005) Differential impact of environmental stresses on the pea mitochondrial proteome. Mol Cell Proteomics 4:1122–1133
Tonge R, Shaw J, Middleton B, Rowlinson R, Rayner S, Young J, Pognan F, Hawkins E, Currie I, Davison M (2001) Validation and development of fluorescence two-dimensional differential gel electrophoresis proteomics technology. Proteomics 1:377–396
Ukaji N, Kuwabara C, Takezawa D, Arakawa K, Fujikawa S (2004) Accumulation of pathogenesis-related (PR) 10/Bet v 1 protein homologues in mulberry (Morus bombycis Koidz.) tree during winter. Plant Cell Environ 27:1112–1121
Uemura M, Tominaga Y, Nakagawara C, Shigematsu S, Minami A, Kawamura Y (2006) Responses of the plasma membrane to low temperatures. Physiol Plant 126:81–89
Wang ZP, Yang PZ, Fan BF, Chen ZX (1998) An oligo selection procedure for identification of sequence-specific DNA-binding activities associated with the plant defense response. Plant J 16:515–522
Wei H, Dhanaraj AL, Rowland LJ, Fu Y, Krebs SL, Arora R (2005) Comparative analysis of expressed sequence tags from cold-acclimated and non-acclimated leaves of Rhododendron catawbiense Michx. Planta 221:406–416
Weiser CJ (1970) Cold resistance and injury in woody plants. Science 169:1269–1278
Welling A, Kaikuranta P, Rinne P (1997) Photoperiodic induction of dormancy and freezing tolerance in Betula pubescens. Involvement of ABA and dehydrins. Physiol Plant 100:119–125
Welling A, Moritz T, Palva ET, Junttila O (2002) Independent activation of cold acclimation by low temperature and short photoperiod in hybrid aspen. Plant Physiol 129:1633–1641
Welling A, Rinne P, Vihera-Aarnio A, Kontunen-Soppela S, Heino P, Palva ET (2004) Photoperiod and temperature differentially regulate the expression of two dehydrin genes during overwintering of birch (Betula pubescens Ehrh.). J Exp Bot 55:507–516
Westram A, Lloyd JR, Roessner U, Riesmeier JW, Kossmann J (2002) Increases of 3-phosphoglyceric acid in potato plants through antisense reduction of cytoplasmic phosphoglycerate mutase impairs photosynthesis and growth, but does not increase starch contents. Plant Cell Environ 25:1133–1143
Wisniewski M, Arora R (1993) Adaptation and response of fruit trees to freezing temperatures. In: Biggs AR (ed) Cytology, Histology and histochemistry of fruit diseases. CRC Press, Boca Raton, Florida, pp 299–320
Wisniewski ME, Arora R (2000) Structural and biochemical aspects of cold hardiness in woody plants. In: Jain M, Minocha S (eds) Molecular biology of woody plants, vol. 2. Kluwer, Dordrecht, pp 419–438
Wisniewski M, Arora R, Artlip T (1995) Seasonal patterns of dehydrin in bark tissue of eight species of woody plants. Hortsc 30:851
Wisniewski M, Close TJ, Artlip T, Arora R (1996) Seasonal patterns of dehydrins and 70-kDa heat-shock proteins in bark tissues of eight species of woody plants. Physiol Plant 96:496–505
Wisniewski M, Basset C, Gusta LV (2003) An overview of cold hardiness in woody plants: seeing the forest through the trees. Plant J 38:952–959
Wisniewski ME, Basset CL, Renaut J, Farrel RE Jr, Tworkoski T, Artlip TS (2006) Differential regulation of two dehydrin genes from peach (Prunus persica) by photoperiod, low temperature and water deficit. Tree Physiol 26:575–584
Witters E, Laukens K, Deckers P, van Dongen W, Esmans E, Van Onckelen H (2003) Fast liquid chromatography coupled to electrospray tandem mass spectrometry peptide sequencing for cross-species protein identification. Rap Comm Mass Spec 17:2188–2194
Woodward EI, Williams BG (1987) Climate and plant distribution at global and local scales. Plant Ecology 69:189–197
Xu XY, Bewley JD, Greenwood JS (2000) Cloning and characterization of an 18 kiloDalton protein in the roots of the perennial weed Taraxacum officinale Weber (Dandelion) which has allergen- and pathogenesis-related protein properties. Plant Cell Environ 23:1227–1236
Zimmerli L, Métraux JP, Mauch-Mani B (2000) b-Aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea. Plant Physiol 126:517–523
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Sederoff
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental material
Summary of the identified proteins, differentially regulated by LT and/or SD or by a combination of both factors, as determined by the ANCOVA. aAccession numbers are issued from ExPASY website (SWISS_Prot database, http://www.expasy.org), bscore = Mascot-MOWSE score, cnumber of peptides matched in databases, dtotal ion score = score calculated by weighting ion scores for all individual peptides matched to a given protein. e P value of the ANCOVA two-ways analysis: in green cells, the abundance values showing an increase; in orange cells, the abundance values showing a decrease. (DOC 292 KB)
Rights and permissions
About this article
Cite this article
Renaut, J., Hausman, JF., Bassett, C. et al. Quantitative proteomic analysis of short photoperiod and low-temperature responses in bark tissues of peach (Prunus persica L. Batsch). Tree Genetics & Genomes 4, 589–600 (2008). https://doi.org/10.1007/s11295-008-0134-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-008-0134-4