Abstract
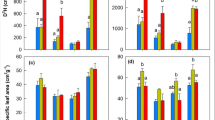
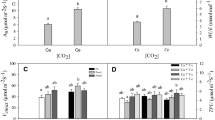
The short-term effects of two levels of air temperature (ambient and warmed) and light (full light and ca. 10% of full light regimes) on the early growth and physiology of Picea asperata and Abies faxoniana seedlings was determined using open-top chambers (OTC). The OTC manipulation increased mean air temperature and soil surface temperature by 0.51°C and 0.34°C under the 60-year plantation, and 0.69°C and 0.41°C under the forest opening, respectively. Warming, with either full-light or low-light conditions, generally caused a significant increase in plant growth, biomass accumulation, and stimulated photosynthetic performance of P. asperata seedlings. However, the warming of A. faxoniana seedlings only significantly increased their growth under low-light conditions, possibly as a result of photoinhibition caused by full light, which may shield and/or impair the effects of warming manipulation, per se, on the growth and physiological performance of A. faxoniana seedlings. In response to warming, P. asperata seedlings allocated relatively more biomass to roots and A. faxoniana more to foliage under similar environments. This might provide A. faxoniana with an adaptive advantage when soil moisture was not limiting and an advantage to P. asperata if substantial moisture stress occurred. Warming markedly increased the efficiency of PSII in terms of the increase in F v/F m and photosynthetic pigment concentrations for the two conifer seedlings, but the effects of warming were generally more pronounced under low-light conditions than under full-light conditions. On balance, this study suggested that warming had a beneficial impact on the early growth and development of conifer seedlings, at least in the short term. Consequently, warming may lead to changes in forest regeneration dynamics and species composition for subalpine coniferous ecosystems under future climate change.




Similar content being viewed by others
References
Aerts R, Cornelissen JHC, Dorrepaal E (2006) Plant performance in a warmer world: general responses of plants from cold, northern biomes and the importance of winter and spring events. Plant Ecol 182:65–77
Aiken RM, Smucker AJM (1996) Root system regulation of whole plant growth. Annu Rev Phytopathol 25:325–346
Awada T, Radoglou K, Fotelli MN, Constantinidou HIA (2003) Ecophysiology of seedlings of three Mediterranean pine species in contrasting light regimes. Tree Physiol 23:33–41
Bergh J, Linder S (1999) Effects of soil warming during spring on photosynthetic recovery in boreal Norway spruce stands. Global Change Biol 5:245–253
Bilger W, Fisahn J, Brummet W, Kossmann J, Willmitzer L (1995) Violaxanthin cycle pigment contents in potato and tobacco plants with genetically reduced photosynthetic capacity. Plant Physiol 108:1479–1486
Cai TB, Dang QL (2002) Effects of soil temperature on parameters of a coupled photosynthesis–stomatal conductance model. Tree Physiol 22:819–827
Cornelissen JHC, Castro Diez P, Hunt R (1996) Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. J Ecol 84:755–765
Danby RK, Hik DS (2007) Responses of white spruce (Picea glauca) to experimental warming at a subarctic alpine treeline. Global Change Biol 13:437–451
Domisch T, Finér L, Lehto T (2002) Growth, carbohydrate and nutrient allocation of Scots pine seedlings after exposure to simulated low soil temperature in spring. Plant Soil 246:75–86
Gielen B, de Boeck HJ, Lemmens CMHM, Valcke R, Nijs I, Ceulemans R (2005) Grassland species will not necessarily benefit from future elevated air temperatures: a chlorophyll fluorescence approach to study autumn physiology. Physiol Plantarum 1:52–63
Hamerlynck EP, Knapp AK (1996) Photosynthetic and stomatal responses to high temperature and light in two oaks at the western limit of their range. Tree Physiol 16:557–566
Havranek WM, Tranquillini W (1995) Physiological processes during winter dormancy and their ecological significance. In: Smith WK, Hinckley TM (eds) Ecophysiology of coniferous forests. Academic Press, San Diego, California, pp 95–124
Hikosaka K, Kato MC, Hirose T (2004) Photosynthetic rates and partitioning of absorbed light energy in photoinhibited leaves. Physiol Plantrum 121:699–708
Hirose T, Werger MJA (1987) Nitrogen use efficiency in instantaneous and daily photosynthesis of leaves in the canopy of a Solodage alissima stand. Physiol Plantarum 70:215–222
Hollister RD, Webber PJ (2000) Biotic validation of small open-top chambers in a tundra ecosystem. Global Change Biol 6:835–842
Hyvönen R, Agren GI, Linder S, Persson T, Cotrufo MF, Ekblad A, Freeman M, Grelle A, Janssens IA, Jarvis PG, Kellomaki S, Lindroth A, Loustau D, Lundmark T, Norby RJ, Oren R, Pilegaard K, Ryan MG, Sigurdsson BD, Stromgren M, van Oijen M, Wallin G (2007) The likely impact of elevated CO2, nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytol 173:463–480
IPCC (2001) Climate change 2001: the scientific basis. Cambridge University Press, Cambridge, UK
Kauppi P, Posch M (2004) Sensitivity of boreal forests to possible climatic warming. Clim Change 7:45–54
Kennedy AD (1995) Simulated climate change: are passive greenhouses a valid microcosm for testing the biological effects of environmental perturbations? Global Change Biol 1:29–42
Kimmins JP (1987) Forest ecology. Macmillan, New York
Kolek J, Kozinka V (1991) Physiology of the plant root system. Kluwer, Dordrecht, The Netherlands
Kullman L (2002) Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. J Ecol 90:68–77
Lambers H, Chapin III FS, Pons TL (1998) Plant physiological ecology. Springer, New York
Lemmens CMHM, de Boeck HJ, Gielen B, Bossuyt H, Malchair S, Carnol M, Merckx R, Nijs I, Ceulemans R (2006) End-of-season effects of elevated temperature on ecophysiological processes of grassland species at different species richness levels. Environ Exp Bot 56:245–254
Lewis JD, Olszyk D, Tingey DT (1999) Seasonal patterns of photosynthetic light response in Douglas-fir seedlings subjected to elevated atmospheric CO2 and temperature. Tree Physiol 19:243–252
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic Press, New York, pp 350–382
Lloret F, Peñuelas J, Estiarte M (2004) Experimental evidence of reduced diversity of seedlings due to climate modification in a Mediterranean-type community. Global Change Biol 10:248–258
Loik ME, Still CJ, Huxman TE, Harte J (2004) In situ photosynthetic freezing tolerance for plants exposed to a global warming manipulation in the Rocky Mountains, Colorado, USA. New Phytol 162:331–341
Loveys BR, Scheurwater I, Pons TL, Fitter AH, Atkin OK (2002) Growth temperature influences the underlying components of relative growth rate: an investigation using inherently fast- and slow-growing plant species. Plant Cell Environ 25:975–988
Marion GM, Henry GHR, Freckman DW, Johnstone J, Jones G, Jones MH, Levesque E, Molau U, Molgaard P, Parsons AN, Svoboda J, Virginia RA (1997) Open-top designs for manipulating field temperature in high-latitude ecosystems. Global Change Biol 3(Suppl 1):20–32
Mitchell AK, Arnott JT (1995) Effects of shade on the morphology and physiology of amabilis fir and western hemlock seedlings. New Forests 10:79–98
Mortensen LV (1994) Effects of carbon dioxide concentration on assimilate partitioning, photosynthesis and transpiration of Betula pendula Roth. and Picea abies (L.) Karst. seedlings at two temperatures. Acta Agr Scand B—S P 44:164–169
Norby RJ, Edwards NT, Riggs JS, Abner CH, Wullschleger SD, Gunderson CA (1997) Temperature-controlled open-top chambers for global change research. Global Change Biol 3:259–267
Ormrod DP, Lesser VM, Olszyk DM, Tingey DT (1999) Elevated temperature and carbon dioxide affect chlorophylls and carotenoids in Douglas-fir seedlings. Int J Plant Sci 160:529–534
Peters RL, Lovejoy TL (1992) Global warming and biological diversity. Yale University Press, New Haven, Connecticut
Price MV, Waser NM (1998) Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology 79:1261–1271
Rosenqvist E, van Kooten O (2003) Chlorophyll fluorescence: a general description and nomenclature. In: DeEll JR, Toivonen PMA (eds) Practical applications of chlorophyll fluorescence in plant biology. Kluwer, Dordrecht, The Netherlands, pp 31–77
Saxe H, Ellsworth DS, Heath J (1998) Tree and forest functioning in an enriched CO2 atmosphere. New Phytol 139:395–436
Schulze ED (1983) Root–shoot interactions and plant life forms. Neth J Agric Sci 4:291–303
Schwarz PA, Fahey TJ, Dawson TE (1997) Seasonal air and soil temperature effects on photosynthesis in red spruce (Picea rubens) saplings. Tree Physiol 17:187–194
Vapaavuori EM, Rikala R, Ryyppö A (1992) Effects of root temperature on growth and photosynthesis in conifer seedlings during shoot elongation. Tree Physiol 3:217–230
Wang K-Y, Kellomäki S, Zha T (2003a) Modifications in photosynthetic pigments and chlorophyll fluorescence in 20-year-old pine trees after a four-year exposure to carbon dioxide and temperature elevation. Photosynthetica 41:167–175
Wang K-Y, Yang WQ, Kellomäki S (2003b) Ecological processes and sustainable mechanisms of the subalpine coniferous forests. World Sci Tech Res Dev 5:17–24
Acknowledgments
This study was sponsored by the Key Program of the National Natural Science Foundation of China (No. 30530630), “Knowledge Innovation Engineering” of the Chinese Academy of Sciences (No. KZCX2-XB02-02), and the Talent Plan of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yin, H.J., Liu, Q. & Lai, T. Warming effects on growth and physiology in the seedlings of the two conifers Picea asperata and Abies faxoniana under two contrasting light conditions. Ecol Res 23, 459–469 (2008). https://doi.org/10.1007/s11284-007-0404-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-007-0404-x