Abstract
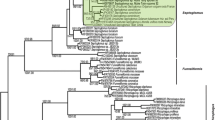
Asymbiotic seedling propagation and introduction of seedlings into a natural habitat were achieved for Cephalanthera falcata. For immature seeds collected 65 days after pollination, high germination rate (av. 50%) was achieved on Hyponex agar medium plates. Root development occurred in about 10% of the protocorms 5 months after seed sowing. Rooted protocorms were transferred to a culture bottle containing 100 ml of the Hyponex agar medium and incubated continually. In about 30% of the transferred individuals, shoot height reached 1.5–2 cm 8 months after the transfer. After acclimatization in wet vermiculite at 4°C for 6 months, 135 individuals were planted in a natural stand of C. falcata in mid February 2001. Shoot appearance rate was 44.4% at the first year and flowering was noted in some plants. At the fifth year, shoots with an average height of 21.6 cm still appeared in four plants, and flowering was noted in three of them. Colonization of mycorrhizal fungi was examined in two of them as well as one wild plant, in which the mycorrhizal fungi were identified to be in Thelephoraceae or Russulaceae. These fungi are known to form ectomycorrhiza with trees, and thus a tripartnership symbiosis consisting of C. falcata, mycorrhizal fungi and trees was suggested. The involvement of ectomycorrhizal fungi might be the reason for the low survival rate in the field experiment, because the distribution of ectomycorrhizal fungi relevant to this orchid is assumed to be uneven. The possibility of introducing artificially propagated orchids into natural habitats was discussed.






Similar content being viewed by others
References
Abadie J-C, Puttsepp U, Gebauer G, Faccio An, Bonfante P, Selosse M-A (2006) Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and nonphotosynthetic individuals. Can J Bot 84:1462–1477
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Arditti J (1967) Factors affecting the germination of orchid seeds. Bot Rev 33:1–97
Arditti J (1982) Orchid biology: reviews and perspectives. Cornell University Press, Ithaca
Arditti J, Michaud JD, Oliva AP (1981) Seed germination of North American orchid I. Native California and related species of Calypso, Epipactis, Goodyla, Piperia and Plantanthera. Bot Gaz 142:442–453
Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ (2004) Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc R Soc Lond B Biol Sci 271:1799–1806
Ballard WW (1987) Sterile propagation of Cypripedium regina from seeds. Am Orchid Soc Bull 56:935–946
Dearnaley JDW, Brocque AFL (2006) Molecular identification of the primary root fungal endophytes of Dipodium hamiltonianum (Orchidaceae). Aust J Bot 54:487–491
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gardes M, Bruns TD (1996) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above-and below-ground views. Can J Bot 74:1572–1583
Girlanda M, Selosse MA, Cafasso D, Brilli F, Delfine S, Fabbian R, Ghignone S, Pinelli P, Segreto R, Loreto F, Cozzolino S, Perotto S (2006) Inefficient photosynthesis in the Mediterranean orchid Limodorum abortivum is mirrored by specific association to ectomycorrhizal Russulaceae. Mol Ecol 15:491–504
Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse MA (2005) Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytol 166:639–653
Kano K (1968) Acceleration of the germination of so-called hard-to-germination orchid seeds. Am Orchid Soc Bull 37:690–698
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kitamura S, Murata G, Koyama T (1964) Colored illustrations of herbaceous plants of Japan (Monocotyledoneae), vol III. Hoikusha, Osaka
Knudson L (1921) La germinacion no simbiotica de las semillas de orquideas. Bol Real Soc Española Hist Nat 21:250–260
Knudson L (1922) Nonsymbiotic germination of orchid seeds. Bot Gaz 73:1–25
Koljalg U (1992) Mycorrhiza formed by basidiospores of Tomentella crinalis on Pinus sylvestris. Mycol Res 96:215–220
Leake JR (1994) Tansley review no. 69 the biology of myco-heterotrophic (saprophytic) plants. New Phytol 127:171–216
McCormick MK, Whigham DF, O’Neill J (2004) Mycorrhizal diversity in photosynthetic terrestrial orchids. New Phytol 163:425–438
Mitsuda S (2003) Cephalanthera falcata (in Japanese) In: Matsui M et al (eds) Red data book of Kyoto Prefecture 2003, popular edn. Sunrise Press, Kyoto, p 143
Miyoshi K (2006) Possibilities and problems on techniques of seed propagation of Cypripedium spp. for their conservation and revegetation (in Japanese). In: Kobayashi T, Kuramoto N (eds) Handbook of revegetation for biodiversity conservation. Chizinshokan, Tokyo, pp 105–115
Nagashima T (1982a) Studies on the seed germination and embryogenesis in Bletilla striata Rchb. f. and Calanthe discolor Lindl (in Japanese with English summary). J Jpn Soc Hort Sci 51:82–93
Nagashima T (1982b) Studies on the seed germination and embryogenesis in Cymbidium goeringii Rchb. f. and Paphiopedilum insigne var. sanderae Rchb. f. (in Japanese with English summary). J Jpn Soc Hort Sci 51:94–105
Nagashima T (1984) On the seed germination and embryogenesis in Calanthe aristulifera Rchb. f., Calanthe izu-insularis Ohwi et. Satomi and Calanthe amamiana Fukuyama (in Japanese with English summary). J Jpn Soc Hort Sci 53:176–186
Nagashima T (1985) On the seed germination and embryogenesis in Calanthe sieboldii Decne., Calanthe elmeri Ames and Calanthe venusta Schltr (in Japanese with English summary). J Jpn Soc Hort Sci 54:231–241
Nagashima T (1989) Embryogenesis, seed formation and immature seed germination in vitro in Ponerorchis graminifolia Reichb. f. (in Japanese with English summary). J Jpn Soc Hort Sci 58:187–194
Nagashima T (1993) Studies on relationship between embryogenesis and germination in Orchidaceae (in Japanese with English summary). J Jpn Soc Hort Sci 62:581–594
Nagashima T (1994) Studies on seed germination and subsequent development in Orchidaceae (in Japanese with English summary). J Jpn Soc Hort Sci 63:139–149
Nagayoshi T, Fukuoka N, Miyake S, Harada H, Takamoto O, Hamano N (1999) Propagation of a rare plant species, Cymbidium nipponicum (Franch. et. Savat.) Makino I (in Japanese). Hyogo Seibutsu 11:259–264
Page RDM (1996) An application to display phylogenetic trees on personal computers. Comp Appl Biosci 12:357–358
Rasmussen HN (2002) Recent developments in the study of orchid mycorrhiza. Plant Soil 244:149–163
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sawa K, Taneda M, Fujimori R (1979) Studies on germination of orchids native to Japan. I. Germination of orchids native to Shikoku Island (in Japanese). Proc Jpn Soc Hort Sci Conf:278–279
Taylor DL, Bruns TD (1997) Independent, specialized invasion of ectomycorrhizal mutualism by two nonphotosynthetic orchids. Proc Natl Acad Sci USA 94:4510–4515
Taylor DL, Bruns TD (1999) Population, habitat and genetic correlates of mycorrhizal specialization in the ‘cheating’ orchids Corallorhiza maculata and C. mertensiana. Mol Ecol 8:1719–1732
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequencing weighting, position sequence gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Yamazaki J, Miyoshi K (2006) In vitro asymbiotic germination of immature seed and formation of protocorm by Cephalanthera falcata (Orchidaceae). Ann Bot 98:1197–1206
Acknowledgments
Some parts of this study, seed harvest, seed germination, and protocorm culture, were funded by Kansai Electric Power Co., Inc. We are grateful to Dr. Chihiro Tanaka, Graduate School of Agriculture, Kyoto University, for instruction in molecular analysis and Dr. Takahiro Yagame, Graduate School of Science and Technology, Chiba University, for help in root sampling. We would like to express our gratitude to Dr. Makoto Ogawa for support extended to this study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yamato, M., Iwase, K. Introduction of asymbiotically propagated seedlings of Cephalanthera falcata (Orchidaceae) into natural habitat and investigation of colonized mycorrhizal fungi. Ecol Res 23, 329–337 (2008). https://doi.org/10.1007/s11284-007-0381-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-007-0381-0