Abstract
Drought severity and duration are expected to increase as a result of ongoing global climate change. Therefore, finding solutions to help plants to deal with drought stress and to improve growth in the face of limited water resources is critical. In this study, a drought tolerant- plant growth promoting endophytic bacterium was isolated from Aloe vera roots. It was identified as Sphingobacterium changzhouense based on 16S rRNA gene sequencing and was deposited into NCBI database with accession number (ON944028). The effect of S. changzhouense inoculation on maize growth under drought stress was investigated. The results revealed that inoculation significantly (p ≤ 0.05) enhanced root and shoot elongation by 205 and 176.19% respectively. Photosynthesis rate, stomatal conductance and water use efficiency were improved in inoculated plants. interestingly, inoculation resulted in significant increase in total chlorophyll, total carbohydrates, proline, total proteins, total phenolics and total flavonoids by 64, 31.5, 25.1, 75.07, 83.7 and 65.4% respectively. Total antioxidant capacity of inoculated plants (51.2 mg/g FW) was higher than that of non-inoculated plants (11.87 mg/g FW), which was found to be positively correlated to the levels of phenolics and flavonoids. Our finding suggests that S. changzhouense could be used to improve crop growth and assist plants to resist drought stress in arid agricultural lands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crop growth and production in many parts of the world have been negatively affected by global climate change, which has resulted in an increase in drought and extreme temperature periods (Grinnan et al. 2013). Drought stress is one of the most critical issues impacting plant growth, development, and production (Mir et al. 2012; Prasanna 2012). It has a variety of effects on plants, including reducing water and nutrient uptake, restricting photosynthesis, and disrupting plasma membranes (Ge et al. 2012). Drought-affected arable lands on the planet have doubled in recent years (Isendahl and Schmidt 2006).
Cereal crops have a significant role in human and animal food systems and significantly contribute to global food security (Zmaic et al. 2007). Maize (Zea mays L.) is an important multipurpose economic cereal crop of the world (Harris et al. 2007). It ranks second after wheat and is equivalent to rice. It participates in the human diet, animal feed, fodder, and bioenergy production (Nyakurwa et al. 2017). Maize plants are highly oversensitive to drought conditions that hamper the growth and yield (Lobell et al. 2011; Zafar-ul-Hye et al. 2014).
Until now, a technique aimed at creating drought-tolerant cultivars has been employed to alleviate the detrimental impacts of drought stress on crops, but it comes with its own set of obstacles, including time consuming and labor cost (Ashraf 2010; Eisenstein 2013). Therefore, the development of microbial-based approaches to mitigate drought stress is of an interest. Currently, plant-associated microorganisms have received attention for enhancing crop stress resistance (Marulanda et al., 2009; Yang et al. 2009). Many beneficial microorganisms have capacity to produce a wide range of enzymes and metabolites help plants to tolerate drought stress (Kim et al. 2009; Pineda et al. 2013; Chauhan et al. 2015). Among these microorganisms are endophytic bacteria that inhabit the internal tissues of plants without causing any adverse effect on the host plant (Ryan et al. 2008). It was reported that the inoculation of plants with endophytic bacteria resulted in water and nutrient uptake enhancement, transpiration regulation, phytohormones induction, antioxidative and photosynthetic improvement, thereby ensuring plant survival under stress conditions (Marulanda et al. 2010; Marasco et al. 2012).
Aloe vera is perennial, succulent plant belongs to the family Asphodelaceae (Liliaceae). It grows in hot, dry climates and has various medicinal benefits (Surjushe et al. 2008). It was selected for this study because it is a drought tolerant plant, and whereas endophytes can confer habitat-specific stress tolerance to their hosts (Rodriguez et al. 2008), we expected that bacteria associated with aloe vera may have a role in alleviating drought stress in plants. Although some Sphingobacterium spp., such as Sphingobacterium pakistanensis and Sphingobacterium sp. BHU-AV3, have been shown to promote plant development under various stress conditions, no studies have been performed on Sphingobacterium changzhouense (Ahmed et al. 2014; Vaishnav et al. 2020). As a result, this is the first study to investigate the role of Sphingobacterium changzhouense in stimulating plant growth under drought stress. This study aims to evaluate the effect of inoculation with the endophytic Sphingobacterium changzhouense isolated from Aloe vera on maize growth under drought stress.
Materials and methods
Isolation and identification of endophytic bacteria
Aloe vera root samples were collected from Aswan University, Egypt (39.59°N 32.82°E). Samples were immediately surface sterilized using 5% sodium hypochlorite (1 min), then 70% ethanol (1 min) and washed three times with sterilized distilled water. Samples were homogenized in sterilized saline solution and filtered. 1 mL of the filtrate was spread into trypticase soy and nutrient agar plates. Plates were incubated at 37 °C with daily observation for 72 h. The selected isolate was coded as Alv and sent to Korea Solgent Lab for molecular identification by 16S rRNA gene sequencing. The obtained sequence was subjected to blast analysis using NCBI website (https://www.ncbi.nlm.nih.gov/) and the percent of similarity with other reference sequences in NCBI database was determined. The present sequence was introduced to NCBI and an access number has been obtained. The MEGA X software was used for the construction of a phylogenetic tree (Kumar et al. 2018).
Assay of drought tolerance of the isolate
The drought tolerance by the isolate was determined according to Saad and Abo-Koura (2018). The isolate was grown in nutrient broth supplemented with different concentrations of polyethylene glycol 6000 i.e., 0, 10, 20 and 30% at 37 °C with shaking (150 rpm) for 72 h. Then, the optical density was measured by spectrophotometer at 600 nm.
Assay of temperature tolerance of the isolate
To determine the effect of temperature on the growth of the isolate, 250-mL flasks contained 50 mL of sterilized nutrient broth were inoculated with 100 µL of standard inoculum (108 CFU/mL). Flasks were incubated at different temperatures i.e., 40, 45, 50 and 55 °C for 72 h. The optical density was measured by spectrophotometer at 600 nm.
Determination of plant growth-promoting activities of the isolate
Indole acetic acid (IAA) production
The bacterial strain was grown in conical flasks contained 100 mL nutrient broth supplemented with 1 g of L-tryptophan (Sigma-Aldrich). Standard inoculum containing about 108 CFU/mL was prepared from 3-day-old bacterial culture using McFarland method (McFarland, 1907). Flasks were inoculated with 1 mL of the inoculum and incubated with shaking (150 rpm) for 72 h at 37 °C. Salkowski method was used to evaluate IAA in the supernatant after centrifuging the culture (Glickmann and Dessaux 1995).
Gibberellic acid (GA3) production
To evaluate the gibberellic acid production by the strain, the method described by Berríos et al. (2004) was followed. Briefly, in 10 mL volumetric flask, 1 mL of bacterial supernatant was vigorously mixed with 1 mL absolute ethanol and 8 mL HCl (3.75 M). Absorbance of the mixture was recorded at 254 nm for 2 min in 20 s interval. The gibberellic acid standard curve was used to calculate the gibberellic acid content.
Exopolysaccharide (EPS) production
The method of Naseem and Bano (2014) was followed to estimate the production of EPS by the isolate. The isolate was cultured in mineral salt medium contained (%): K2HPO4, 12.6, KH2PO4, 18.2, NH4NO3, 10, MgSO4.7H2O, 1, MnSO4, 0.6, CaCl2.2H2O, 1, FeSO4.2H2O, 0.06, sodium molybdate, 1, NaCl, 1.5 and glucose, 0.2). Culture was incubated in a shaker incubator (150 rpm) for 7 days at 37 °C. Then, culture was centrifuged, and supernatant was mixed with two volumes of cold absolute ethanol. The precipitate was collected, washed, dried and weighted.
Phosphate solubilization
The strain was spot inoculated on Pikovskaya’s agar plates. Plates were incubated at 37 °C for 7 days. Diameter of clear halo zones appeared around the growth was measured (Karpagam and Nagalakshmi 2014).
Effect of bacterial inoculation on maize growth under drought stress
The effect of isolate Alv inoculation on the growth of maize under drought stress was investigated. Maize grains (TWC 321) were obtained from the Agricultural Research Center, Egypt. Grains were surface sterilized with sodium hypochlorite (5%) for 3 min, then washed three times with sterilized distilled water. Sterilized grains were soaked in 50 mL bacterial suspension (108 CFU/mL) of isolate Alv and sterilized distilled water (Control) for 3 h. Inoculated grains were sown in plastic pots containing autoclaved soil mixture (2:1, v/v) of clay and sand supplemented with bacterial inoculum (100 mL/Kg soil). Control pots received sterilized distilled water. Pots were divided into three groups as follow: the first group (control under normal irrigation) is non-inoculated subjected to water regime 90% field capacity, while the second group (control under drought stress) is non-inoculated subjected to water regime 35% field capacity, and the third group (treatment under drought stress) is inoculated with isolate Alv and subjected to water regime 35% field capacity. Pots were kept under normal climatic conditions. After two months of sowing, plants were collected for evaluation of growth and physiological parameters as well as biochemical constituents. The experiment was repeated twice with five replicates.
Measurement of growth parameters
Root length, shoot length as well as fresh and dry biomasses were measured for randomly selected plants.
Measurement of gas-exchange and photosynthesis parameters
The net photosynthesis rate (Pn), transpiration rate (E), leaf stomatal conductance (C) and leaf water-use efficiency (WUE) were measured for randomly selected fully expanded healthy leaves. Measurements were carried out in controlled leaf chamber using infrared gas analyzer (IRGA, CI 340) photosynthesis system (CID Bio-Science, Inc.). Levels of photosynthetically active radiation (PAR) and incoming air CO2 were set at 1500 μmol m−2 s −1 and 360 ppm respectively. Relative humidity and temperature in the leaf chamber were 50% and 26 ± 0.1 °C. Data was recorded between 12:57 and 2:30 PM.
Measurement of biochemical constituents
Total chlorophyll
Chlorophyll content was measured from the expanded leaves of maize plants. About 1 g leaves was homogenized and extracted with 10 mL of 80% acetone (Arnon 1949). The contents of chlorophylls a and b in the filtrates were estimated by reading the absorbance at 645 and 663 nm respectively using UV–visible spectrophotometer (UVmini-1240, Shimadzu Corporation, Japan). The total chlorophyll was calculated according to the following formula: Total chlorophyll (mg/g FW) = 20.2 A645 + 8.02 A663 (Porra et al. 1989).
Total carbohydrates
Morris (1948) method was used to measure the content of carbohydrates in the seedlings. Briefly, 1 g of plant materials were hydrolyzed for 2 h at 100 °C using HCl (4 N). The hydrolysates were cooled and filtered. 9 mL of 2% (w/v) anthrone reagent prepared in concentrated H2SO4 was added to 1 mL of sample filtrates. Then, the reaction mixtures were heated for 7 min and cooled. The absorbance was measured at 630 nm using UV–visible spectrophotometer (model UVmini-1240, Shimadzu Corporation, Japan). The content of carbohydrates (mg/ g fresh weight) was calculated using a standard curve of glucose.
Proline content
The method of Bates et al. (1973) was followed to estimate proline content. 1 g of plant tissue was homogenized in 10 mL of 3% sulphosalicylic acid and centrifuged. 2 mL of acid ninhydrin reagent (2.5 g ninhydrin dissolved in 40 mL of orthophosphoric acid (6 M) and 60 mL of glacial acetic acid) was mixed with 2 mL of the supernatant and 2 mL of glacial acetic acid. The reaction mixture was heated at 100 °C for 1 h. After cooling, 4 mL of toluene was added. Absorbance was measured at 520 nm. Proline content was quantified using proline standard curve.
Total proteins
Total proteins content of seedlings was estimated using Lowry assay (Lowry et al. 1951). Reagent A was prepared by dissolving 2 g of Na2CO3 in 100 mL NaOH (0.1 N) and reagent B was prepared by dissolving 0.5 g of CuSO4⋅5H2O in 100 mL sodium- potassium tartarate (1%). Reagent C was prepared by mixing 50 mL of reagent A with 1 mL of reagent B. 1 g of plant material was mixed with 5 mL of reagent C and was incubated at room temperature for 15 min. Then, 0.5 mL of Folin–Ciocalteau reagent was added to the mixture and was left for 30 min. The absorbance against the blank was measured at 700 nm. Bovine Serum Albumin (Sigma-Aldrich) was used to construct the calibration curve. The content of protein was expressed as mg/g fresh weight.
Total phenolics, total flavonoids and total antioxidant capacity (TAC)
One gram of fresh plant tissue was macerated in 40 mL of 80% methanol, vortexed and placed in water bath at 60 °C for 1 h. The extracts were centrifuged and filtered. The obtained filtrates were used for estimation of total phenolics, total flavonoids and total antioxidant capacity. Total phenolics content was evaluated by Folin-Ciocalteu reagent method according to Singleton et al. (1999) and expressed as mg gallic acid equivalents per gram of fresh weight. The content of total flavonoids was estimated using the aluminum chloride colorimetric assay and was expressed as mg quercetin equivalents per gram of fresh weight (Zhishen et al. 1999). Phosphomolybdenum assay was used to estimate the total antioxidant capacity and was expressed as mg ascorbic acid equivalents per gram of fresh weight using ascorbic acid as a reference (Prieto et al. 1999).
Statistical analysis
Data obtained were subjected to one-way analysis of variance (ANOVA) using Minitab 18 software. Values are means ± standard errors of five biological replicates (n = 5) obtained from two independent experiments. The significant differences between means were computed by Tukey's HSD test at p ≤ 0.05.
Results
Isolation and identification
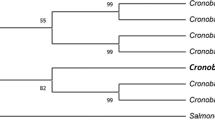
The comparative sequence analysis of isolate Alv with NCBI GenBank database using BLAST tool showed that isolate Alv belongs to genus Sphingobacterium and exhibited the highest similarity percent with Sphingobacterium changzhouense strain N7 (NR135709) (Fig. 1). The 16S rRNA gene sequence of the present isolate was deposited to NCBI GenBank under the accession number (ON944028).
Drought tolerance of the isolate
Isolate Alv was screened for its drought tolerance by growing it at different concentrations of polyethylene glycol. It was grown at polyethylene glycol concentrations ranged from 0 to 30% (Fig. 2).
Temperature tolerance of the isolate
The isolate was grown at different temperatures to evaluate its temperature tolerance. The results showed that it could tolerate up to 45 °C, above this the growth was declined (Fig. 3).
Plant growth-promoting activities of the isolate
The present isolate was screened for plant growth promoting activities including indole acetic acid, gibberellic acid, and exopolysaccharide production as well as phosphate solubilization. Significant activities were noted (Table 1).
Effect of bacterial inoculation on maize growth under drought stress
The effect of the inoculation with isolate Alv on the growth and physiological parameters as well as biochemical constituents of maize grown under drought stress was evaluated.
Growth parameters
Table 2 shows the growth characteristics for non-inoculated and inoculated plants, including root and shoot length and fresh and dry biomass. Drought stress was found to be detrimental to maize growth, resulting in significant reductions in all assessed growth parameters. Inoculation with isolate Alv, on the other hand, had a statistically significant favorable effect on maize growth as compared to non-inoculated plants.
Gas-exchange and photosynthesis parameters
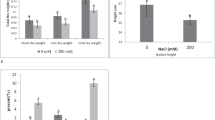
Drought stress significantly (p ≤ 0.05) decreased the net photosynthesis rate, stomatal conductance, and water-use efficiency in non-inoculated plants. Significantly, inoculation with isolate Alv enhanced gas-exchange and photosynthesis in maize plants under drought stress (Fig. 4). Photosynthesis rate, stomatal conductance, and water use efficiency in inoculated plants increased by 104.7, 73.8 and 194.2% compared with non-inoculated plants respectively. On the other hand, inoculated plants exhibited significant decrease in transpiration rate by 57% compared with non-inoculated plants under drought stress.
Biochemical constituents
Differences in biochemical constituents between inoculated and non-inoculated maize plants under drought stress were represented by (Fig. 5). Under stress condition, the leaf chlorophyll content of plants was decreased by 16.26% as compared with their respective control (under normal irrigation). Inoculation with isolate Alv increased chlorophyll content in stressed plants by 64% compared with stressed non-inoculated plants. Inoculated maize plants showed significant increase (p ≤ 0.05) in total carbohydrates under drought stress compared with non-inoculated plants (Fig. 5). Moreover, the contents of proline, proteins, phenolics and flavonoids were increased under drought stress in inoculated plants by quantum of 25.1, 75.07, 83.7 and 65.4% respectively over the non-inoculated. Under drought stress, it was noted that the antioxidant capacity of the inoculated plants (51.2 mg/g FW) was higher than that of non-inoculated plants (11.87 mg/g FW), which was positively correlated with the contents of phenolics and flavonoids.
Differences in total chlorophyll, total carbohydrates, proline content, total proteins, total phenolics, total flavonoids and total antioxidant capacity (TAC) (mg/g FW) between inoculated and non-inoculated plants. a, b, c indicates significant differences (p ≤ 0.05) between inoculated and non-inoculated plants. FC field capacity
Discussion
Drought is a major challenge to crop development and productivity in many places of the world (Vinocur and Altman 2005; Naveed et al. 2014). Drought is expected to cause major plant development problems for crops on more than half of the world's arable lands by 2050 (Vinocur and Altman 2005). Therefore, crop drought tolerance enhancement is regarded as the most pressing concern. Consequently, the effect of Sphingobacterium changzhouense Alv inoculation on maize growth under drought stress was investigated in this study.
S. changzhouense Alv was isolated from the roots of drought-adapted plant Aloe vera. It exhibited significant drought and temperature tolerance (Figs. 2, 3). This may be because the bacterial cell can protect its structures and organelles under heat- drought conditions through accumulation of compatible solutes such as proline, glycine betaine and trehalose as well as exopolysaccharides that increase thermostability of enzymes, inhibits proteins thermal denaturation, and maintain membrane integrity (Welsh 2000; Conlin and Nelson 2007; Bérard et al. 2015).
Interestingly, the whole growth of maize under drought stress was improved upon inoculation with isolate Alv compared to drought stressed non-inoculated plants (Fig. 6). Drought stressed inoculated maize plants showed an increase in root and shoot lengths by 205 and 176.19% compared to drought stressed non-inoculated plants (Table 2). This may be because isolate Alv produces phytohormones like indole acetic acid and gibberellic acid (Table) that stimulate cell division as well as elongation of roots and stems (Glick 1995). Improvement of root and shoot systems enables plants undergoing drought to increase water and nutrient uptake as well as photosynthesis efficiency and consequently enhances plant growth (Timmusk et al. 2014). The improvement of root and shoot growth of maize via bacterial inoculation was previously reported (Vardharajula et al. 2011; Naveed et al. 2014).
Photosynthesis is an important physico-chemical process that directly effects on plant growth and biomass production (Yang et al. 2014). Drought stress alters physiological processes, it disrupts photosynthetic pigments, reduces gas exchange and stomatal function, and consequently causes reductions in net photosynthesis (Keyvan 2010). In the present study, photosynthetic parameters in non-inoculated plants were strongly affected by drought stress (Fig. 4). On the other hand, the inoculation with isolate Alv significantly (p ≤ 0.05) increased gas exchange and photosynthetic parameters. Interestingly, inoculation increased the net photosynthesis rate under drought stress by 104.7% over the non-inoculated plants (Fig. 4). This may be because inoculation enhances stomatal conductance that regulates gas exchange and subsequently enhances photosynthesis (Kusumi et al. 2012).
Water use efficiency is one of the most crucial factors limiting crop production worldwide and its improvement is of major concern with drought problems (Waraich et al. 2011). In the present study, water use efficiency was significantly (p ≤ 0.05) increased in drought-stressed inoculated plants compared with drought-stressed non-inoculated plants (Fig. 4). High water use efficiency combined with stomatal conductance showed that isolate Alv inoculation can be beneficial for water transportation through plants and can help plants keep their stomata open. Therefore, the response may be an important mechanism for maize plants to adapt to drought stress. The obtained results are like those obtained in previous studies where enhancement of water use efficiency and stomatal conductance upon inoculation of maize with Pseudomonas spp. was reported (Sandhya et al. 2010).
The content of photosynthetic pigments is an important physiological indicator for drought tolerance (Pour-Aboughadareh et al. 2020). In this study, the total chlorophyll content was significantly increased by 64% in plants upon inoculation with isolate Alv (Fig. 5).
In the present study, it was noted that the total carbohydrates content was significantly increased in inoculated plants compared with non-inoculated plants during drought stress (Fig. 5). The accumulation of carbohydrates is part of a wider mechanism for plant surviving during drought stress that plays a key role in the regulation of carbon metabolism (Praxedes et al. 2005). Increases in carbohydrates content attributable to bacterial inoculation under drought stress conditions was previously documented (Heidari et al. 2011; Kalita et al. 2015; Omara et al. 2017).
Proline is one of the most crucial compatible solutes that accumulates in drought-stressed plants (Farooq et al. 2008). It contributes to stabilizing proteins and membranes as well as scavenging free radicals (Ashraf and Foolad 2007; Hayat et al. 2012). In this study, the proline level accumulated by inoculated maize plants under drought stress was higher by 76.89% than those accumulated by non-inoculated plants. Increase in proline levels upon bacterial inoculation has been demonstrated in maize under drought stress (Sandhya et al. 2010; Vardharajula et al. 2011; Naseem and Bano 2014).
The total proteins significantly (p ≤ 0.05) increased in inoculated plants compared with non-inoculated plants under drought stress (Fig. 5). Increasing in protein content under drought stress was previously reported (Qaseem et al. 2019). On the other hand, the total phenolics and total flavonoids showed significant increases in the inoculated plants comparing to non-inoculated plants (Fig. 5). These compounds were found to prevent tissues from oxidative damage and enable plants to tolerate stresses (Pazoki 2015; Ilangumaran and Smith 2017; Nawaz and Bano 2020). Our results agreed with those obtained by Jha (2017) who reported that phenolics and flavonoids were enhanced in PGPB-inoculated maize under normal and stress conditions. Furthermore, the total antioxidant capacity was significantly (p ≤ 0.05) increased in inoculated plants compared with the non-inoculated plants under drought stress (Fig. 5). In this study, positive correlation was noted between total antioxidant capacity and phenolics as well as flavonoids contents. The presence of positive correlation between phenolics, flavonoids and antioxidant activities has been previously reported (Ghasemzadeh et al. 2012; Baharfar et al. 2015). Improvement of antioxidant capacity mediated by bacterial inoculation under drought stress was previously documented (Erdogan et al. 2016; Nawaz and Bano 2020).
Conclusion
In the current study, the endophyte Sphingobacterium changzhouense Alv was isolated from Aloe vera. It showed multiple plant growth-promoting activities as well as drought and temperature tolerance. The results of this study provide evidence that inoculation with S. changzhouense can enhance drought tolerance of maize through improving plant growth and physio-biochemical status. So, the application of endophyte inoculation approach should be encouraged in dryland farming in order to improve crop growth and drought resistance.
References
Ahmed I, Ehsan M, Sin Y, Paek J, Khalid N, Hayat R et al (2014) Sphingobacterium pakistanensis sp. Nov., a novel plant growth promoting rhizobacteria isolated from rhizosphere of Vigna mungo. Antonie Van Leeuwenhoek 105(2):325–333. https://doi.org/10.1007/s10482-013-0077-0
Arnon DI (1949) Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta Vulgaris. Plant Physiol 24(1):1–15. https://doi.org/10.1104/pp.24.1.1
Ashraf M (2010) Inducing drought tolerance in plants: some recent advances. Biotechnol Adv 28:169–183. https://doi.org/10.1016/j.biotechadv.2009.11.005
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59(2):206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
Baharfar R, Rahmani Z, Mohseni M, Azimi R (2015) Evaluation of the antioxidant and antibacterial properties of ethanol extracts from berries, leaves and stems of Hedera pastuchovii Woron ex. Grossh. Nat Prod Res 29(22):2145–2148. https://doi.org/10.1080/14786419.2014.994211
Bates LS, Waldran RO, Terare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–208. https://doi.org/10.1007/BF00018060
Bérard A, Ben Sassi M, Kaisermann A, Renault P (2015) Soil microbial community responses to heat wave components: drought and high temperature. Clim Res 66:243–264. https://doi.org/10.3354/cr01343
Berríos J, Illanes A, Aroca G (2004) Spectrophotometric method for determining gibberellic acid in fermentation broths. Biotechnol Lett 26:67–70. https://doi.org/10.1023/B:BILE.0000009463.98203.8b
Chauhan H, Bagyaraj DJ, Selvakumar G, Sundaram SP (2015) Novel plant growth promoting rhizobacteria-prospects. Appl Soil Ecol 95:38–53. https://doi.org/10.1016/j.apsoil.2015.05.011
Conlin LK, Nelson HCM (2007) The natural osmolytetrehalose is a positive regulator of the heat-induced activity of yeast heat shock transcription factor. Mol Cell Biol 27(4):1505–1515. https://doi.org/10.1128/MCB.01158-06
Eisenstein M (2013) Discovery in a dry spell. Nature 501:S7–S9. https://doi.org/10.1038/501S7a
Erdogan U, Cakmakci R, Varmazyari A, Turan M, Erdogan Y, Kitir N (2016) Role of inoculation with multi-trait rhizobacteria on strawberries under water deficit stress. Zemdirbyste-Agric 103(1):67–76. https://doi.org/10.13080/z-a.2016.103.009
Farooq M, Basra SMA, Wahid A, Cheema ZA, Cheema MA, Khaliq A (2008) Physiological role of exogenously applied glycine betaine in improving drought tolerance of fine grain aromatic rice (Oryza sativa L.). J Agron Crop Sci 194:325–333. https://doi.org/10.1111/j.1439-037X.2008.00323.x
Ge T, Sui F, Bai L, Tong C, Sun N (2012) Effects of water stress on growth, biomass partitioning, and water-use efficiency in summer maize (Zea mays L.) throughout the growth cycle. Acta Physiol Plant 34(3):1043–1053. https://doi.org/10.1007/s11738-011-0901-y
Ghasemzadeh A, Azarifar M, Soroodi O, Jaafar HZE (2012) Flavonoid compounds and their antioxidant activity in extract of some tropical plants. J Med Plants Res 6(13):2639–2643. https://doi.org/10.5897/JMPR11.1531
Glick B (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41(2):109–117. https://doi.org/10.1139/m95-01
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61(2):793–796. https://doi.org/10.1128/aem.61.2.793-796.1995
Grinnan R, Carter TE, Johnson MT (2013) Effects of drought, temperature, herbivory, and genotype on plant–insect interactions in soybean (Glycine max). Arthropod-Plant Interact 7:201–215. https://doi.org/10.1007/s11829-012-9234-z
Harris A, Rashid G, Miaj M, Arif M, Shah H (2007) On-farm seed priming with zinc sulphate solution-A cost-effective way to increase the maize yields of resources-poor farmers. Field Crops Res 102(2):119–127. https://doi.org/10.1016/j.fcr.2007.03.005
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments. Plant Signal Behav 7(11):1456–1466. https://doi.org/10.4161/psb.21949
Heidari M, Mousavinik SM, Golpayegani A (2011) Plant growth-promoting rhizobacteria (PGPR) effect on physiological parameters and mineral uptake in basil (Ociumum basilicm L.) under water stress. ARPN J Agric Biol Sci 6:6–11
Ilangumaran G, Smith DL (2017) Plant growth-promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci 8:1768. https://doi.org/10.3389/fpls.2017.01768
Isendahl N, Schmidt G (2006) Drought in the Mediterranean. WWF Policy Proposals, WWF Report, Madrid
Jha Y (2017) Cell water content and lignification in maize regulated by rhizobacteria under salinity. Braz J Biol Sci 4(7):9–18. https://doi.org/10.21472/bjbs.040702
Kalita M, Bharadwaz M, Dey T, Gogoi K, Dowarah P (2015) Developing novel bacterial based bioformulation having PGPR properties for enhanced production of agricultural crops. Indian J Exp Biol 53(1):56–60
Karpagam T, Nagalakshmi PK (2014) Isolation and characterization of phosphate solubilizing microbes from agricultural soil. Int J Curr Microbiol Appl Sci 3(3):601–614
Keyvan S (2010) The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J Anim Plant Sci 8:1051–1060
Kim YC, Glick BR, Bashan Y, Ryu CM (2009) Enhancement of plant drought tolerance by microbes. In: Aroca R (ed) Plant responses to drought stress. Springer-Verlag, Berlin, pp 383–412
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Kusumi K, Hirotsuka S, Kumamaru T, Iba K (2012) Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J Exp Bot 63(15):5635–5644. https://doi.org/10.1093/jxb/ers216
Lobell DB, Bänziger M, Magorokosho C, Vivek B (2011) Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat Clim Chang 1:42–45. https://doi.org/10.1038/nclimate1043
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Marasco R, Rolli E, Ettoumi B, Vigani G, Mapelli F, Borin S, Zocchi G (2012) A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS ONE 7(10):e48479. https://doi.org/10.1371/journal.pone.0048479
Marulanda A, Barea J-M, Azcón R (2009) Stimulation of plant growth and drought tolerance by native microorganisms (AM Fungi and Bacteria) from dry environments: mechanisms related to bacterial effectiveness. J Plant Growth Regul 28:115–124. https://doi.org/10.1007/s00344-009-9079-6
Marulanda A, Azcón R, Chaumont F, Ruiz-Lozano JM, Aroca R (2010) Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 232(2):533–543. https://doi.org/10.1007/s00425-010-1196-8
Mir RR, Zaman-Allah M, Sreenivasulu N, Trethowan R, Varshney RK (2012) Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor Appl Genet 125:625–645. https://doi.org/10.1007/s00122-012-1904-9
Morris DL (1948) Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 107:254–255
Naseem H, Bano A (2014) Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J Plant Interact 9(1):689–701. https://doi.org/10.1080/17429145.2014.902125
Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A (2014) Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD 17. Environ Exp Bot 97:30–39. https://doi.org/10.1016/j.envexpbot.2013.09.014
Nawaz S, Bano A (2020) Effects of PGPR (Pseudomonas sp.) and Ag-nanoparticles on enzymatic activity and physiology of cucumber. Recent Pat Food Nutr Agric 11(2):124–136. https://doi.org/10.2174/2212798410666190716162340
Nyakurwa CS, Gasura E, Mabasa S (2017) Potential for quality protein maize for reducing protein energy undernutrition in maize dependent Sub-Saharan African countries: a review. Afr Crop Sci J 25(4):521–537. https://doi.org/10.4314/acsj.v25i4.9
Omara A, Hauka F, Afify A, Nour El-Din M, Kassem M (2017) The role of some PGPR strains to biocontrol Rhizoctonia Solani in soybean and enhancement the growth dynamics and seed yield. Environ Biodivers Soil Secur 1(2017):47–59. https://doi.org/10.21608/jenvbs.2017.993.1003
Pazoki A (2015) Evaluation of flavonoids and phenols content of wheat under different lead, PGPR and mycorrhiza levels. BFIJ 7:309–315
Pineda A, Dicke M, Pieterse CMJ, Pozo MJ (2013) Beneficial microbes in a changing environment: are they always helping plants deal with insects? Funct Ecol 27:574–586. https://doi.org/10.1111/1365-2435.12050
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectrometry. Biochim Biophys Acta Bioenerg 975(3):384–394. https://doi.org/10.1016/S0005-2728(89)80347-0
Pour-Aboughadareh A, Etminan A, Abdelrahman M, Siddique KHM, Tran LSP (2020) Assessment of biochemical and physiological parameters of durum wheat genotypes at the seedling stage during polyethylene glycol-induced water stress. Plant Growth Regul 92:81–93. https://doi.org/10.1007/s10725-020-00621-4
Prasanna BM (2012) Diversity in global maize germplasm: characterization and utilization. J Biosci 37:843–855. https://doi.org/10.1016/j.wace.2014.04.004
Praxedes SC, DaMatta FM, Loureiro MEG, Ferrao MA, Cordeiro AT (2005) Effects of long-term soil drought on photosynthesis and carbohydrate metabolism in mature robusta coffee (Coffea canephora Pierre var. kouillou) leaves. Environ Exp Bot 56(3):263–273. https://doi.org/10.1016/j.envexpbot.2005.02.008
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269(2):337–341. https://doi.org/10.1006/abio.1999.4019
Qaseem MF, Qureshi R, Shaheen H (2019) Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat (Triticum aestivum L.) genotypes varying in sensitivity to heat and drought stress. Sci Rep 9:6955. https://doi.org/10.1038/s41598-019-43477-z
Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim Y, Redman RS (2008) Stress tolerance in plants via habitat-adapted symbiosis. Inter Soc Microb Ecol 2:404–416
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. Fems Microbiol Lett 278(1):1–9. https://doi.org/10.1111/j.1574-6968.2007.00918.x
Saad M, Abo-Koura H (2018) Improvement of sorghum (Sorghum bicolor L. Moench) growth and yield under drought stress by inoculation with bacillus cereus and foliar application of potassium silicate. Environ Biodivers Soil Secur 2(2018):205–221. https://doi.org/10.21608/jenvbs.2019.6790.1045
Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B (2010) Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul 62:21–30. https://doi.org/10.1007/s10725-010-9479-4
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in enzymology, vol 299. Elsevier, Amsterdam, pp 152–178
Surjushe A, Vasani R, Saple DG (2008) Aloe vera: a short review. Indian J Dermatol 53(4):163–166. https://doi.org/10.4103/0019-5154.44785
Timmusk S, Abd El-Daim IA, Copolovici L, Tanilas T, Kannaste A, Behers L, Niinemets U (2014) Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 9:e96086. https://doi.org/10.1371/journal.pone.0096086
Vaishnav A, Singh J, Singh P, Rajput RS, Singh HB, Sarma BK (2020) Sphingobacterium sp. BHU-AV3 induces salt tolerance in tomato by enhancing antioxidant activities and energy metabolism. Front Microbiol 11:443. https://doi.org/10.3389/fmicb.2020.00443
Vardharajula S, Ali SZ, Grover M, Reddy G, Bandi V (2011) Drought-tolerant plant growth promoting Bacillus spp.: effect on growth osmolytes, and antioxidant status of maize under drought stress. J Plant Interact 6(1):1–14. https://doi.org/10.1080/17429145.2010.535178
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16(2):123–132. https://doi.org/10.1016/j.copbio.2005.02.001
Waraich EA, Ahmad R, Ashraf MY, Saifullah A, Ahmad M (2011) Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agric Scand Sect B 61:291–304. https://doi.org/10.1080/09064710.2010.491954.Wa
Welsh DT (2000) Ecological significance of compatible solute accumulation by microorganisms: from single cells to global climate. FEMS Microbiol Rev 24(3):263–290. https://doi.org/10.1111/j.1574-6976.2000.tb00542.x
Yang J, Kloepper JW, Ryu C (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14(1):1–4. https://doi.org/10.1016/j.tplants.2008.10.004
Yang PM, Huang QC, Qin GY, Zhao SP, Zhou JG (2014) Different drought-stress responses in photosynthesis and reactive oxygen metabolism between autotetraploid and diploid rice. Photosynthetica 52(2):193–202. https://doi.org/10.1007/s11099-014-0020-2
Zafar-ul-Hye M, Farooq HM, Zahir ZA, Hussain M, Hussain A (2014) Application of ACC deaminase containing rhizobacteria with fertilizer improves maize production under drought and salinity stress. Int J Agric Biol 16:591–596
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Zmaic K, Loncaric R, Sudaric T (2007) economic efficiency of cereal crops production by application of pam. Cereal Res Commun 35(2):693–696
Acknowledgements
We introduce our sincere thanks and gratitude to the Botany Department, Faculty of Science, Aswan University for supporting and providing the requirements of scientific research.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
N.Sh.A.H. contributed to the study design. Material preparation, Methodology, data collection and analysis, and wrote the main manuscript text, and U.M.A-R. contributed to data analysis, read, and reviewed the fnal manuscript.
Corresponding author
Ethics declarations
Competing interests
We declare that we have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hagaggi, N.S.A., Abdul-Raouf, U.M. Drought-tolerant Sphingobacterium changzhouense Alv associated with Aloe vera mediates drought tolerance in maize (Zea mays). World J Microbiol Biotechnol 38, 248 (2022). https://doi.org/10.1007/s11274-022-03441-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03441-y