Abstract
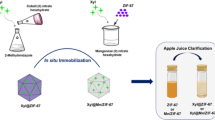
d-allulose is a rare low-calorie sugar that has many fundamental biological functions. d-allulose 3-epimerase from Agrobacterium tumefaciens (AT-DAEase) catalyzes the conversion of d-fructose to d-allulose. The enzyme has attracted considerable attention because of its mild catalytic properties. However, the bioconversion efficiency and reusability of AT-DAEase limit its industrial application. Magnetic metal–organic frameworks (MOFs) have uniform pore sizes and large surface areas and can facilitate mass transport and enhance the capacity for enzyme immobilization. Here, we successfully encapsulated cobalt-type AT-DAEase into the cobalt-based magnetic MOF ZIF-67@Fe3O4 using a self-assembly strategy. We confirmed the immobilization of enzyme AT-DAEase and characterized the enzymatic properties of the MOF-immobilized AT-DAEase@ZIF-67@Fe3O4. The AT-DAEase@ZIF-67@Fe3O4 nanoparticles had higher catalytic activity (65.1 U mg−1) and bioconversion ratio (38.1%) than the free AT-DAEase. The optimal conditions for maximum enzyme activity of the AT-DAEase@ZIF-67@Fe3O4 nanoparticles were 55 °C and pH 8.0, which were significantly higher than those of the free AT-DAEase (50 °C and pH 7.5). The AT-DAEase@ZIF-67@Fe3O4 nanoparticles displayed significantly improved thermal stability and excellent recycling performance, with 80% retention of enzyme activity at a temperature range of 45–70 °C and > 45% of its initial activity after eight cycles of enzyme use. The AT-DAEase@ZIF-67@Fe3O4 nanoparticles have great potential for large-scale industrial preparation of d-allulose by immobilizing cobalt-type AT-DAEase into magnetic MOF ZIF-67@Fe3O4.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Dedania SR, Patel MJ, Patel DM, Akhani RC, Patel DH (2017) Immobilization on graphene oxide improves the thermal stability and bioconversion efficiency of d-psicose 3-epimerase for rare sugar production. Enzyme Microb Technol 107:49–56. https://doi.org/10.1016/j.enzmictec.2017.08.003
Dicosimo R, Mcauliffe JC, Poulose AJ, Bohlmann G (2013) Industrial use of immobilized enzymes. Chem Soc Rev 42(15):6437–6474. https://doi.org/10.1039/C3CS35506C
Franssen MC, Steunenberg P, Scott EL, Zuilhof H, Sanders JP (2013) Immobilised enzymes in biorenewables production. Chem Soc Rev 42(15):6491–6533. https://doi.org/10.1039/C3CS00004D
Hammes GG, Wu CW (1971) Regulation of enzyme activity. The activity of enzymes can be controlled by a multiplicity of conformational equilibria. Science 172(3989):1205–1211. https://doi.org/10.1126/science.172.3989.1205
Itoh H, Okaya H, Khan AR, Tajima S, Hayakawa S, Izumori K (1994) Purification and characterization of d-tagatose 3-epimerase from Pseudomonas sp. ST-24. Biosci Biotech Bioch 58(12):2168–2171. https://doi.org/10.1271/bbb.58.2168
Kaneti YV, Dutta S, Hossain MSA, Shiddiky MJA, Tung KL, Shieh FK, Tsung CK, Wu KC, Yamauchi Y (2017) Strategies for improving the functionality of zeolitic imidazolate frameworks: tailoring nanoarchitectures for functional applications. Adv Mater 29(38):1700213. https://doi.org/10.1002/adma.201700213
Kim K, Kim HJ, Oh DK, Cha SS, Rhee S (2006a) Crystal structure of d-psicose 3-epimerase from Agrobacterium tumefaciens and its complex with true substrate d-fructose: a pivotal role of metal in catalysis, an active site for the non-phosphorylated substrate, and its conformational changes. J Mol Biol 361(5):920–931. https://doi.org/10.1016/j.jmb.2006.06.069
Kim HJ, Hyun EK, Kim YS, Lee YJ, Oh DK (2006b) Characterization of an Agrobacterium tumefaciens d-psicose 3-epimerase that converts d-fructose to d-psicose. Appl Environ Microbiol 72(2):981–985. https://doi.org/10.1128/AEM.72.2.981-985.2006
Li P, Moon SY, Guelta MA, Harvey SP, Hupp JT, Farha OK (2016) Encapsulation of a nerve agent detoxifying enzyme by a mesoporous zirconium metal–organic framework engenders thermal and long-term stability. J Am Chem Soc 138(26):8052–8055. https://doi.org/10.1021/jacs.6b03673
Lian X, Fang Y, Joseph E, Wang Q, Li J, Banerjee S, Lollar C, Wang X, Zhou HC (2017) Enzyme-MOF (metal-organic framework) composites. Chem Soc Rev 46(11):3386–3401. https://doi.org/10.1039/C7CS00058H
Liang K, Ricco R, Doherty CM, Styles MJ, Bell S, Kirby N, Mudie S, Haylock D, Hill AJ, Doonan CJ, Falcaro P (2015) Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat Commun 6:7240. https://doi.org/10.1038/ncomms8240
Lim B, Kim H, Oh D (2009) A stable immobilized d-psicose 3-epimerase for the production of d-psicose in the presence of borate. Process Biochem 44(8):822–828. https://doi.org/10.1016/j.procbio.2009.03.017
Liu J, Sun Z, Deng Y, Zou Y, Li C, Guo X, Xiong L, Gao Y, Li F, Zhao D (2009) Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew Chem Int Ed Engl 48(32):5875–5879. https://doi.org/10.1002/anie.200901566
Mao S, Cheng X, Zhu Z, Chen Y, Li C, Zhu M, Liu X, Lu F, Qin HM (2020) Engineering a thermostable version of d-allulose 3-epimerase from Rhodopirellula baltica via site-directed mutagenesis based on B-factors analysis. Enzyme Microb Technol 132:109441. https://doi.org/10.1016/j.enzmictec.2019.109441
Matsuo T, Suzuki H, Hashiguchi M, Izumori K (2002) d-psicose is a rare sugar that provides no energy to growing rats. J Nutr Sci Vitaminol 48(1):77–80. https://doi.org/10.3177/jnsv.48.77
Matyska L, Kovár J (1985) Comparison of several non-linear-regression methods for fitting the Michaelis-Menten equation. Biochem J 231(1):171–177
Meshkat S, Kaliaguine S, Rodrigue D (2020) Comparison between ZIF-67 and ZIF-8 in Pebax® MH-1657 mixed matrix membranes for CO2 separation. Sep Purif Technol 235:116150–116150. https://doi.org/10.1016/j.seppur.2019.116150
Mu W, Chu F, Xing Q, Yu S, Zhou L, Jiang B (2011) Cloning, expression, and characterization of a d-psicose 3-epimerase from Clostridium cellulolyticum H10. J Agric Food Chem 59(14):7785–7792. https://doi.org/10.1021/jf201356q
Patel SN, Singh V, Sharma M, Sangwan RS, Singhal NK, Singh SP (2018) Development of a thermo-stable and recyclable magnetic nanobiocatalyst for bioprocessing of fruit processing residues and d-allulose synthesis. Bioresour Technol 247:633–639. https://doi.org/10.1016/j.biortech.2017.09.112
Patel SN, Kaushal G, Singh SP (2020) A novel d-allulose 3-epimerase gene from the metagenome of a thermal aquatic habitat and d-allulose production by bacillus subtilis whole-cell catalysis. Appl Environ Microbiol 86(5):e02605-e2619. https://doi.org/10.1128/AEM.02605-19
Patel SN, Kaushal G, Singh SP (2021) d-allulose 3-epimerase of Bacillus sp. origin manifests profuse heat-stability and noteworthy potential of d-fructose epimerization. Microb Cell Fact 20(1):60. https://doi.org/10.1186/s12934-021-01550-1
Pei X, Zhang H, Meng L, Xu G, Yang L, Wu J (2013) Efficient cloning and expression of a thermostable nitrile hydratase in Escherichia coli using an auto-induction fed-batch strategy. Process Biochem 48(12):1921–1927. https://doi.org/10.1016/j.procbio.2013.09.004
Pei X, Wu Y, Wang J, Chen Z, Liu W, Su W, Liu F (2020) Biomimetic mineralization of nitrile hydratase into a mesoporous cobalt-based metal-organic framework for efficient biocatalysis. Nanoscale 12(2):967–972. https://doi.org/10.1039/c9nr06470b
Sheldon RA, Woodley JM (2018) Role of biocatalysis in sustainable chemistry. Chem Rev 118(2):801–838. https://doi.org/10.1021/acs.chemrev.7b00203
Sun Y, Hayakawa S, Ogawa M, Fukada K, Izumori K (2008) Influence of a rare sugar, d-psicose, on the physicochemical and functional properties of an aerated food system containing egg albumen. J Agric Food Chem 56(12):4789–4796. https://doi.org/10.1021/jf800050d
Talekar S, Ghodake V, Ghotage T, Rathod P, Deshmukh P, Nadar S, Mulla M, Ladole M (2012) Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresour Technol 123:542–547. https://doi.org/10.1016/j.biortech.2012.07.044
Tseng CW, Liao CY, Sun Y, Peng CC, Tzen JT, Guo RT, Liu JR (2014) Immobilization of Clostridium cellulolyticum d-psicose 3-epimerase on artificial oil bodies. J Agric Food Chem 62(28):6771–6776. https://doi.org/10.1021/jf502022w
Wu X, Yang C, Ge J, Liu Z (2015) Polydopamine tethered enzyme/metal-organic framework composites with high stability and reusability. Nanoscale 7(45):18883–18886. https://doi.org/10.1039/c5nr05190h
Yogapriya R, Datta KKR (2020) Porous fluorinated graphene and ZIF-67 composites with hydrophobic-oleophilic properties towards oil and organic solvent sorption. J Nanosci Nanotechnol 20(5):2930–2938. https://doi.org/10.1166/jnn.2020.17465
Zdarta J, Meyer A, Jesionowski T, Pinelo M (2018) A general overview of support materials for enzyme immobilization: characteristics, properties, practical utility. Catalysts 8(2):92. https://doi.org/10.3390/catal8020092
Zhang W, Fang D, Xing Q, Zhou L, Jiang B, Mu W (2013) Characterization of a novel metal-dependent d-psicose 3-epimerase from Clostridium scindens 35704. PLoS ONE 8(4):e62987. https://doi.org/10.1371/journal.pone.0062987
Zheng L, Sun Y, Wang J, Huang H, Geng X, Tong Y, Wang Z (2018) Preparation of a flower-like immobilized d-psicose 3-epimerase with enhanced catalytic performance. Catalysts 8(10):468. https://doi.org/10.3390/catal8100468
Zhu Y, Men Y, Bai W, Li X, Zhang L, Sun Y, Ma Y (2012) Overexpression of d-psicose 3-epimerase from Ruminococcus sp. in Escherichia coli and its potential application in d-psicose production. Biotechnol Lett 34(10):1901–1906. https://doi.org/10.1007/s10529-012-0986-4
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20190610) and the 111 Project (111-2-06).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, K., Liu, CL., Yang, Y. et al. Immobilization of d-allulose 3-epimerase into magnetic metal–organic framework nanoparticles for efficient biocatalysis. World J Microbiol Biotechnol 38, 144 (2022). https://doi.org/10.1007/s11274-022-03330-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03330-4