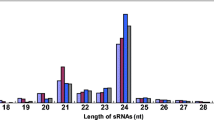
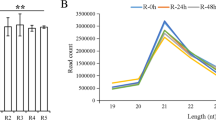
Abstract
Endophytic Streptomyces sp. SSD49 inhibited eight pathogens, including the human opportunistic pathogenic microorganisms, the plant pathogenic fungi and bacteria. The growth of soybeans, tomatoes, peppers and Populus tomentosa seedings inoculated with SSD49 are remarkably promoted. Here, we constructed two P. tomentosa seedling microRNA (miRNA) libraries inoculated with (PS30d) and without SSD49 (PC30d) to explore the molecular regulatory roles in the plant response to the beneficial bacteria. Totals of 314 known and 144 novel miRNAs were identified, among which 27 known and 11 novel miRNA had significantly different expression. The targets of up-regulated miR160, miR156, ptc114 and down-regulated miR319 and other differential expressed miRNAs primarily regulated genes encoding transcription factors (auxin response factor, small auxin-up RNA, and GRAS proteins), disease resistance proteins, phytohormone oxidase, and response regulators, which could promote plant growth, influence disease resistance and miRNA biosynthesis in P. tomentosa. This is the first report on the genome-wide identification of biocontrol endophytic Streptomyces inoculation-responsive miRNAs using small RNA sequencing in P. tomentosa and these findings provide new insight into understanding the biocontrol effects of endophytic Streptomyces.
Graphic abstract










Similar content being viewed by others
References
Allen E, Xie Z, Gustafson AM, Carrington JC (2005) microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121(2):207–221
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11(10):R106
Aung B, Gruber MY, Amyot L, Omari K, Bertrand A, Hannoufa A (2015) MicroRNA156 as a promising tool for alfalfa improvement. Plant Biotechnol J 13(6):779–790
Berg G (2009) Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol 84(1):11–18
Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ (2008) miRBase: tools for microRNA genomics. Nucleic Acids Res 36(SI):154–158
Gutierrez L, Bussell JD, Pacurar DI, Schwambach J, Pacurar M, Bellini C (2009) Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 21:3119–3132
Hagen G, Guilfoyle T (2002) Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol 49:373–385
Han J, Xie H, Sun Q, Wang J, Lu M, Wang W, Guo E, Pan J (2014) Bioinformatic identification and experimental validation of miRNAs from foxtail millet (Setariaitalica). Gene 546:367–377
Hivrale V, Zheng Y, Puli COR, Jagadeeswaran G, Gowdu K, Kakani VG, Barakat A, Sunkar R (2016) Characterization of drought-and heat-responsive microRNAs in switchgrass. Plant Sci 242(SI):214–223.
June RR, Aggarwal R (2014) The Use and Abuse of Diagnostic/Classification Criteria. Best Pract Res Clin Rheumatol 28(6):921
Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M (2011) KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res 40:109–114
Kong Y, Zhu Y, Gao C, She W, Lin W, Chen Y, Han N, Bian H, Zhu M, Wang J (2013) Tissue-specific expression of SMALL AUXIN UPRNA41 differentially regulates cell expansion and root meristem patterning in Arabidopsis. Plant Cell Physiol 54(4):609–621
Li R, Li Y, Kristiansen K, Wang J (2008) SOAP: short oligonucleotide alignment program. Bioinformatics 24(5):713–714
Liu Q, Wang H, Hu H, Zhang H (2015) Genome-wide identification and evolutionary analysis of positively selected miRNA genes in domesticated rice. Mol Genet Genomics 290(2):593–602
Liu X, Dou G, Ma Y (2016) Potential of endophytes from medicinal plants for biocontrol and plant growth promotion. J Gen Plant Pathol 82(3):165–173
Lu S, Sun YH, Shi R, Clark C, Li L, Chiang VL (2005) Novel and mechanical stress-responsive microRNAs in Populus trichocarpa that are absent from Arabidopsis. Plant Cell 17(8):2186–2203
Ludwig AA, Romeis T, Jones JD (2004) CDPK-mediated signalling pathways: specificity and cross-talk. J Exp Bot 55(395):181–188
Man MZ, Wang X, Wang Y (2000) POWER_SAGE: comparing statistical tests for SAGE experiments. Bioinformatics 16(11):953–959
Mathews DH, Sabina J, Zuker M, Turner DH (1999) Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol 288(5):911–940
McCormack JE, Hird SM, Zellmer AJ, Carstens BC, Brumfield RT (2013) Applications of next generation sequencing to phylogeography and phylogenetics. Mol Phylogenet Evol 66:526–538
Meyers BC, Axtell MJ, Bartel B, Bartel DP, Baulcombe D, Bowman JL et al (2008) Criteria for annotation of plant MicroRNAs. Plant Cell 20(12):3186–3190
Morozova O, Marra M (2008) Applications of next-generation sequencing technologies in functional genomics. Genomics 92:255–264
Morrissey JP, Dow JM, Mark GL, O’Gara F (2004) Are microbes at the root of a solution to world food production? EMBO Rep 5(10):922–926
Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D (2003) Control of leaf morphogenesis by microRNAs. Nature 425(6955):257–263
Ren Y, Chen L, Zhang Y, Kang X, Zhang Z, Wang Y (2013) Identification and characterization of salt-responsive microRNAs in Populus tomentosa by high-throughput sequencing. Biochimie 95(4):743–750
Rossi M, Trupiano D, Tamburro M, Ripabelli G, Montagnoli A, Chiatante D, Scippa GS (2015) MicroRNAs expression patterns in the response of poplar woody root to bending stress. Planta 242(1):339–351
Santoyo G, Moreno-Hagelsieb G, del Carmen Orozco-Mosqueda M, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99
Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D (2005) Specific effects of microRNAs on the plant transcriptome. Dev Cell 8(4):517–527
Shuai P, Liang D, Zhang Z, Yin W, Xia X (2013) Identification of drought-responsive and novel Populus trichocarpa microRNAs by high-throughput sequencing and their targets using degradome analysis. BMC Genomics 14:233
Sjödin A, Street NR, Sandberg G, Gustafsson P, Jansson S (2009) The Populus genome integrative explorer (PopGenIE): a new resource for exploring the Populus genome. New Phytol 182(4):1013–1025
Stief A, Altmann S, Hoffmann K, Pant BD, Scheible WR, Bäurle I (2014) Arabidopsis miR156 regulates tolerance to recurring environmental stress through SPL transcription factors. Plant Cell 26(4):1792–1807
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16(8):2001–2019
Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U et al. (2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313(5793):1596–1604.
Voinnet O (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136:669–687
Wang JJ, Guo HS (2015) Cleavage of INDOLE-3-ACETIC ACID INDUCIBLE28 mRNA by microRNA847 upregulatesauxin signaling to modulate cell proliferation and lateral organ growth in Arabidopsis. Plant Cell 27(3):574–590
Wang L, Mai YX, Zhang YC, Luo Q, Yang HQ (2010) MicroRNA171c-targeted SCL6-II, SCL6-III, and SCL6-IV genes regulate shoot branching in Arabidopsis. Mol plant 3(5):794–806
Xu Z, Ji A, Song J, Chen S (2016) Genome-wide analysis of auxin response factor gene family members in medicinal model plant Salvia miltiorrhiza. Biol Open 5(6):848–857
Yadav A, Khan Y, Prasad M (2016) Dehydration-responsive miRNAs in foxtail millet: genome-wide identification, characterization and expression profiling. Planta 243(3):749–766
Yin Z, Ke X, Huang D, Gao X, Voegele RT, Kang Z, Huang L (2013). Validation of reference genes for gene expression analysis in Valsa mali var. mali using real-time quantitative PCR. World J Microbiol Biotechnol 29(9):1563-1571.
Yoon EK, Yang JH, Lim J, Kim SH, Kim SK, Lee WS (2010) Auxin regulation of the microRNA390-dependent transacting small interfering RNA pathway in Arabidopsis lateral root development. Nucleic Acids Res 38:1382–1391
Acknowledgements
This research was supported by the Fundamental Research Funds for the Central Universities (2017ZY14, 2016JX03), the National Science and Technology Ministry (2012BAC09B03), the National Natural Science Foundation of China (J1310005) and the Beijing Nova Program (2011033). We thank Prof. Wei He for the contribution of D. gregaria, B. dothidea CFCC 7926/7897 and L. quercina subsp. populi; Prof. Shidong Li for C. parasitica, S. sclerotiorum and P. capsici; and Prof. Deqiang Zhang for P. tomentosa tissue culture seedlings. We thank the professional Scientific and Technical Editing Service (https://www.textcheck.com/text/page/about) for the edition of the final version of the manuscript.
Author information
Authors and Affiliations
Contributions
YM conceived and designed the experiments. YG, XL and GD conducted experiments and analyzed data. WT and YG wrote the manuscript and should be as the first co-authors. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, W., Ge, Y., Liu, X. et al. Identification and characterization of Populus microRNAs in response to plant growth-promoting endophytic Streptomyces sp. SSD49. World J Microbiol Biotechnol 35, 97 (2019). https://doi.org/10.1007/s11274-019-2671-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2671-4