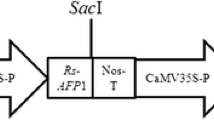
Abstract
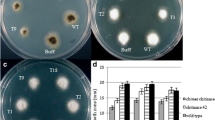
Canola (Brassica napus) plants were co-transformed with two pathogenesis-related protein genes expressing a Trichoderma atroviride chitinase with a chitin-binding domain (chimeric chitinase) and a thaumatin-like protein (tlp) from Oryza sativa conferring resistance to phytopatogenic fungi by Agrobacterium-mediated transformation. The putative transgenic plants were confirmed by PCR. After measuring the specific activity of the chimeric chitinase and glucanase activity for tlp genes, transgenic plants with high specific activity were selected for southern blot analysis to confirm the copy number of the genes. In vitro assays, the antifungal activity of crude extracted protein against Sclerotinia sclerotiorum showed that the inhibition percentage in double transgenic plants was between 55 and 62, whereas the inhibition percentage in single-gene transformants (chimeric chitinase) ranged from 35 to 45 percent. Importantly, in greenhouse conditions, the double transgenic plants showed significant resistance than the single-gene transformant and wild type plants. The results in T2 generation using the intact leaf inoculation method showed that the average lesion diameters were 10, 14.7 and 29 mm for the double transformant, single-gene transformant and non-transgenic plants, respectively. Combined expression of chimeric chitinase and tlp in transgenic plants showed significantly enhanced resistance against S. sclerotiorum than the one that express single-gene transformant plants. These results suggest that the co-expression of chimeric chitinase and tlp can confer enhanced disease resistance in canola plant.









Similar content being viewed by others
References
Acharya K, Pal AK, Gulati A, Kumar S, Singh AK, Ahuja PS (2013) Overexpression of Camellia sinensis thaumatin-like protein, CsTLP in potato confers enhanced resistance to Macrophomina phaseolina and Phytophthora infestans infection. Mol Biotechnol 54:609–622
Alvarez ML, Guelman S, Halford NG, Lustig S, Reggiardo MI, Ryabushkina N, Schewry P, Stein J, Vallejos RH (2000) Silencing of HMW glutenins in transgenic wheat expressing extra HMW subunits. Theor Appl Genet 100:319–327
Anand A, Zhou T, Trick HN, Gill BS, Bockus WW, Muthukrishnan S (2003) Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum. J Exp Bot 54:1101–1111
Banoub J, Boullanger P, Lafont D (1992) Synthesis of oligosaccharids of 2-amino-2-deoxy sugers. Chem Rev 92:1167–1195
Baranski R, Klocke E, Nothnagel T (2008) Chitinase CHIT36 from Trichoderma harzianum enhances resistance of transgenic carrot to fungal pathogens. J Phytopathol 156:513–521
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Broekaert WF, Terras FR, Cammue BP, Vanderleyden J (1990) An automated quantitative assay for fungal growth inhibition. FEMS Microbiol Lett 69:55–59
Broekaert WF et al (1992) Antimicrobial peptides from Amaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin-binding proteins. Biochemistry 31:4308–4314
Chang M-M, Culley D, Choi JJ, Hadwiger LA (2002) Agrobacterium-mediated co-transformation of a pea b-1,3-glucanase and chitinase genes in potato (Solanum tuberosum L. c.v. Russet Burbank) using a single selectable marker. Plant Sci 163:83–89
Chen W, Punja Z (2002) Transgenic herbicide-and disease-tolerant carrot (Daucus carota L.) plants obtained through Agrobacterium-mediated transformation. Plant Cell Rep 20:929–935
Chhikara S, Chaudhury D, Dhankher OP, Jaiwal PK (2012) Combined expression of a barley class II chitinase and type I ribosome inactivating protein in transgenic Brassica juncea provides protection against Alternaria brassicae. Plant Cell, Tissue Organ Cult 108:83–89
Chowdhury S, Basu A, Kundu S (2015) Cloning, characterization, and bacterial over-expression of an Osmotin-like protein gene from Solanum nigrum L. with antifungal activity against three necrotrophic fungi. Mol Biotechnol 57:371–381
De Buck S, Windels P, De Loose M, Depicker A (2004) Single-copy TDNAs integrated at different positions in the Arabidopsis genome display uniform and comparable beta-glucuronidase accumulation levels. Cell Mol Life Sci 61:2632–2645
Demeke T, Hucl P, Båga M, Caswell K, Leung N, Chibbar RN (1999) Transgene inheritance and silencing in hexaploid spring wheat. Theor Appl Genet 99:947–953
Dong X et al (2008) Expressing a gene encoding wheat oxalate oxidase enhances resistance to Sclerotinia sclerotiorum in oilseed rape (Brassica napus). Planta 228:331–340
Grenier J, Potvin C, Trudel J, Asselin A (1999) Some thaumatin-like proteins hydrolyse polymeric β-1, 3-glucans. Plant J 19:473–480
Grenier J, Potvin C, Asselin A (2000) Some fungi express β-1, 3-glucanases similar to thaumatin-like proteins. Mycologia 92(5):841–848
Huang Y, Liu H, Jia Z, Fang Q, Luo K (2012) Combined expression of antimicrobial genes (Bbchit1 and LJAMP2) in transgenic poplar enhances resistance to fungal pathogens. Tree Physiol 32:1313–1320
Jayaraj J, Punja Z (2007) Combined expression of chitinase and lipid transfer protein genes in transgenic carrot plants enhances resistance to foliar fungal pathogens. Plant Cell Rep 26:1539–1546
Joosten MHAJ, Verbakel HM, Nettekoven ME, van Leeuwen J, van der Vossen RTM, de Wit PJGM (1995) The phytopathogenic fungus Cladosporium fulvum is not sensitive to the chitinase and β-1,3-glucanase defence proteins of its host, tomato. Physiol Mol Plant Pathol 46:45–59
Kahl G, Winter P (1995) Plant genetic engineering for crop improvement. World J Microbiol Biotechnol 11:449–460
Kalpana K et al (2006) Engineering sheath blight resistance in elite indica rice cultivars using genes encoding defense proteins. Plant Sci 170:203–215
Kronland W, Stanghellini M (1988) Clean slide technique for the observation of Anastomosis and nuclear condition of Rhizoctonia solarti. Phytopathology 78:820–822
Kumar HGA, Hegde VL, Shetty SM, Venkatesh YP (2013) Characterization and gene cloning of an acidic thaumatin-like protein (TLP 1), an allergen from sapodilla fruit (Manilkara zapota). Allergol Int 62:447–462
Liu J-J, Sturrock R, Ekramoddoullah AK (2010a) The superfamily of thaumatin-like proteins: its origin, evolution, and expression towards biological function. Plant Cell Rep 29:419–436
Liu J-J, Zamani A, Ekramoddoullah AK (2010b) Expression profiling of a complex thaumatin-like protein family in western white pine. Planta 231:637–651
Liu H et al (2011) Transgenic Brassica napus L. lines carrying a two gene construct demonstrate enhanced resistance against Plutella xylostella and Sclerotinia sclerotiorum. Plant Cell, Tissue Organ Cult 106:143–151
Lorito M et al (1998) Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proc Natl Acad Sci USA 95:7860–7865
Mao B, Liu X, Hu D, Li D (2014) Co-expression of RCH10 and AGLU1 confers rice resistance to fungal sheath blight Rhizoctonia solani and blast Magnorpathe oryzae and reveals impact on seed germination. World J Microbiol Biotechnol 30:1229–1238
Maruthasalam S et al (2007) Pyramiding transgenic resistance in elite indica rice cultivars against the sheath blight and bacterial blight. Plant Cell Rep 26:791–804
Matroodi S, Motallebi M, Zamani M, Moradyar M (2013) Designing a new chitinase with more chitin binding and antifungal activity. World J Microbiol Biotechnol 29:1517–1523
Melander M, Kamnert I, Happstadius I, Liljeroth E, Bryngelsson T (2006) Stability of transgene integration and expression in subsequent generations of doubled haploid oilseed rape transformed with chitinase and β-1, 3-glucanase genes in a double-gene construct. Plant Cell Rep 25:942–952
Menu-Bouaouiche L, Vriet C, Peumans WJ, Barre A, Van Damme EJM, Rougé P (2003) A molecular basis for the endo-b1,3-glucanase activity of the thaumatin-like proteins from edible fruits. Biochimie 85:123–131
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nagpure A, Choudhary B, Gupta RK (2014) Chitinases: in agriculture and human healthcare. Crit Rev Biotechnol 34:215–232
Oguchi M, Oguchi MS (1979) Tetraborate concentration on Morman-Elson reaction and an improved method for hexosamine determination. Anal Biochem 98:433–437
Punja ZK (2005) Transgenic carrots expressing a thaumatin-like protein display enhanced resistance to several fungal pathogens. Can J Plant Pathol 27:291–296
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5:69–76
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sharma P, Sharma M, Srivastava M (2014) Heterologous expression and characterization of purified partial endochitinase (ech-42) isolated from Trichoderma harzianum. Afr J Biotechnol 13:2159–2165
Srivastava V, Vasil V, Vasil IK (1996) Molecular characterization of the fate of transgenes in transformed wheat (Triticum aestivum L.). Theor Appl Genet 92:1031–1037
Srivastava M, Gupta SK, Saxena AP, Shittu LAJ, Gupta SK (2011) A review of occurrence of fungal pathogens on significant brassicaceous vegetable crops and their control measures. Asian j Agric Sci 3(2):70–79
Tesfaye M, Denton MD, Samac DA, Vance CP (2005) Transgenic alfalfa secretes a fungal endochitinase protein to the rhizosphere. Plant Soil 269:233–243
Travella S, Ross S, Harden J, Everett C, Snape J, Harwood W (2005) A comparison of transgenic barley lines produced by particle bombardment and Agrobacterium-mediated techniques. Plant Cell Rep 23:780–789
Wang Y, Jones JD, Weller SC, Goldsbrough PB (1991) Expression and stability of amplified genes encoding 5-enolpyruvylshikimate-3-phosphate synthase in glyphosate-tolerant tobacco cells. Plant Mol Biol 17:1127–1138
Wang Q, Zhang Y, Hou Y, Wang P, Zhou S, Ma X, Zhang N (2012) Purification, characterization of a CkChn134 protein from Cynanchum komarovii seeds and synergistic effect with CkTLP against Verticillium dahliae. Protein Sci 21:865–875
Acknowledgments
We would like to acknowledge National Institute of Genetic Engineering and Biotechnology (Project No. 407-M) for providing financial support for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghazadeh, R., Zamani, M., Motallebi, M. et al. Co-transformation of canola by chimeric chitinase and tlp genes towards improving resistance to Sclerotinia sclerotiorum . World J Microbiol Biotechnol 32, 144 (2016). https://doi.org/10.1007/s11274-016-2104-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-016-2104-6