Abstract
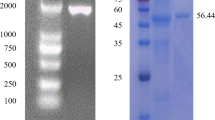
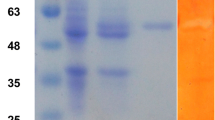
The growing demands of bioenergy has led to the emphasis on novel cellulases to improve efficiency of biodegradation process of plant biomass. Therefore, a thermostable cellulolytic gene (CenC) with 3675 bp was cloned from Clostridium thermocellum and over-expressed in Escherichia coli strain BL21 CodonPlus. It was attested that CenC belongs to glycoside hydrolase family 9 (GH9) with four binding domains, a processive endoglucanase. CenC was purified to homogeneity, producing a single band on SDS-PAGE corresponding to 137.11 kDa, by purification steps of heat treatment combined with ion-exchange chromatography. Purified enzyme displayed optimal activity at pH 6.0 and 70 °C. CenC had a half-life of 24 min at 74 °C, was stable up to 2 h at 60 °C and over a pH range of 5.5–7.5. Enzyme showed high affinity towards various substrates and processively released cellobiose from cellulosic substrates. It efficiently hydrolyzed carboxymethyl cellulose (30 U/mg), β-Glucan Barley (94 U/mg); also showed activity towards p-nitrophenyl-β-d-cellobioside (18 U/mg), birchwood xylan (19 U/mg), beechwood xylan (17.5 U/mg), avicel (9 U/mg), whatman filter paper (11 U/mg) and laminarin (3.3 U/mg). CenC exhibited Km, Vmax, Kcat, Vmax K −1m and Kcat K −1m of 7.14 mM, 52.4 µmol mg−1 min−1, 632.85 s−1, 7.34 min−1 and 88.63, respectively used CMC as substrate. Recombinant CenC saccharified pretreated wheat straw and bagasse to 5.12 and 7.31 %, respectively at pH 7.0 and 45 °C after 2 h incubation. Its thermostability, high catalytic efficiency and independence of inhibitors make CenC enzyme an appropriate candidate for industrial applications and cost-effective saccharification process.




Similar content being viewed by others
References
Bajaj BK, Pangotra H, Wani AM, Sharma P, Sharma A (2009) Partial purification and characterization of a highly thermostable and pH stable endoglucanase from a newly isolated Bacillus strain M-9. Indian J Chem Technol 16:382–387
Bradford MM (1976) A dye binding assay for protein. Anal Biochem 72:248–254
Downing M, Eaton LM, Graham RL, Langholtz MH, Perlack RD, Turhollow AF, Stokes B, Brandt CC (2011) US billion-ton update: the biomass supply for a bioenergy and bioproducts industry. Oak Ridge National Laboratory, Oak Ridge
El-Naghy MA, El-Katatny MS, Attia AA (1991) Degradation of cellulosic materials by Sporotrichum thermophile culture filtrate for sugar production. Int Biodeterioration 27(1):75–79
Gaudin C, Belaich A, Champ S, Belaich JP (2000) CelE, a multidomain cellulase from Clostridium cellulolyticum: a Key Enzyme in the Cellulosome? J Bacteriol 182(7):1910–1915
Gilad R, Rabinovich L, Yaron S, Edward A, Lamed R, Gilbert HJ, Shoham Y (2003) CelI, a noncellulosomal family 9 enzyme from Clostridium thermocellum, is a processive endoglucanase that degrades crystalline cellulose. J Bacteriol 185(2):391–398
Iqbal NMH, Ahmed I, Zia AM, Irfan M (2011) Purification and characterization of the kinetic parameters of cellulase produced from wheat straw by Trichoderma viride under SSF and its detergent compatibility. Adv Biosci Biotechnol 2:149–156
Ja’afaru MI, Fagade OE (2007) Cellulase production and enzymatic hydrolysis of some selected local lignocellulosic substrates by a strain of Aspergillus niger. Res J Bio Sci 2(1):13–16
Kataeva I, Li X-L, Chen H, Ljungdahl LG (1999) Cloning and sequence analysis of a new cellulase gene encoding CelK, a major cellulosome component of Clostridium thermocellum: evidence for gene duplication and recombination. J Bacteriol 181:5288–5295
Kataeva IA, Uversky VN, Brewer JM, Schubot F, Rose JP, Wang BC, Ljungdahl LG (2004) Interactions between immunoglobulin-like and catalytic modules in Clostridium thermocellum cellulosomal cellobiohydrolase CbhA. Protein Eng 17(11):759–769
Khademi S, Guarino LA, Watanabe H, Tokuda G, Meyer EF (2002) Structure of an endoglucanase from termite, Nasutitermes takasagoensis. Acta Crystallogr D Biol Crystallogr 58:653–659
Kibbe WA (2007) OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res 35(Suppl 2):W43–W46. doi:10.1093/nar/gkm234
Kocher GS, Kalra KL (2013) Optimization of pretreatment, enzymatic saccharification and fermentation conditions for bioethanol production from rice straw. Ind J Appl Res 3(5):62–64
Koppram R, Pejó ET, Xiros C, Olsson L (2014) Lignocellulosic ethanol production at high-gravity: challenges and perspectives. Trends Biotechnol 32(1):46–53
Liang C, Fioronic M, Rodriguez-Roperoc F, Xuea Y, Schwanebergc U, Maa Y (2011) Directed evolution of a thermophilic endoglucanase (Cel5A) into highly active Cel5A variants with an expanded temperature profile. J Biotechnol 154:46–53
Liu J, Liu W, Zhao X (2011) Cloning and functional characterization of a novel endo-β-1,4-glucanase gene from a soil-derived metagenomic library. App Microbiol Biotechnol 89:1083–1092
Liu D, Zhang R, Yang X, Zhang Z, Song S, Miao Y, Shen Q (2012) Characterization of a thermostable β-glucosidase from Aspergillus fumigatus Z5, and its functional expression in Pichia pastoris X33. Microb Cell Fact 11:25
Maki M, Leung KT, Qin W (2009) The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int J Biol Sci 5(5):500–516
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Morana A, Esposito A, Maurelli L, Ruggiero G, Ionata E, Rossi M, La Cara L (2008) A novel thermoacidophilic cellulase from Alicyclobacillus acidocaldarius. Protein Pept Lett 15:1017–1021
Narraa M, Dixit G, Divecha J, Kumar K, Madamwar D, Shah AR (2014) Production, purification and characterization of a novel GH 12 family endoglucanase from Aspergillus terreus and its application in enzymatic degradation of delignified rice straw. Int Biodeterior Biodegrad 88:150–161
Parsiegla G, Juy M, Reverbel-Leroy C, Tardif C, Belaich JP, Driguez H, Haser R (1998) The crystal structure of the processive Endocellulase, CelF, of Clostridium cellulolyticum in complex with a thio-oligosaccharide inhibitor at 2.0 A° resolution. EMBO J 17:5551–5562
Pham TH, Quyen DT, Nghiem NM (2012) Purification and properties of an endoglucanase from Aspergillus niger VTCC-F021. Turk J Biol 36:694–701
Reverbel-Leroy C, Pages S, Belaich A, Belaich JP, Tardif C (1997) The processive endocellulase CelF, a major component of the Clostridium cellulolyticum cellulosome, purification and characterization of the recombinant form. J Bacteriol 179:46–52
Riederer A, Takasuka TE, Makino S, Stevenson DM, Bukhman YV, Elsen NL, Fox BG (2011) Global gene expression patterns in Clostridium thermocellum as determined by microarray analysis of chemostat cultures on cellulose or cellobiose. Appl Environ Microbiol 77(4):1243–1253
Sadhu S, Saha P, Sen SK, Mayilraj S, Maiti TK (2013) Production, purification and characterization of a novel thermotolerant endoglucanase (CMCase) from Bacillus strain isolated from cow dung. Springer Plus 2(1):1–10
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor, New York
Selig M, Weiss N, Ji Y (2008) Enzymatic saccharification of lignocellulosic biomass. NREL Ethanol Project CAT Task Laboratory Analytical Procedure #009
Shi R, Li Z, Ye Q, Xu J, Liu Y (2013) Heterologous expression and characterization of a novel thermo-halotolerant endoglucanase Cel5H from Dictyoglomus thermophilum. Bioresour Technol 142:338–344
Shi H, Zhang Y, Wang L, Li X, Li W, Wang F, Li X (2014) Molecular analysis of hyperthermophilic endoglucanase Cel12B from Thermotoga maritima and the properties of its functional residues. BMC Struct Biol 14:8
Silva VF, Arruda PV, Felipe MG, Gonçalves AR, Rocha GJ (2011) Fermentation of cellulosic hydrolysates obtained by enzymatic saccharification of sugarcane bagasse pretreated by hydrothermal processing. J Ind Microbiol Biotechnol 38(7):809–817
Te’o VSJ, Saul DJ, Bergquist PL (1995) CelA, another gene coding for a multidomain cellulase from the extreme thermophile Caldocellum saccharolyticum. Appl Microbiol Biotechnol 43:291–296
Vallander L, Eriksson KE (1985) Enzymic saccharification of pretreated wheat straw. Biotechnol Bioeng 27(5):650–659
Várnai A, Tang C, Bengtsson O, Atterton A, Mathiesen G, Vincent GH (2014) Expression of endoglucanases in Pichia pastoris under control of the GAP promoter. Microb Cell Fact 13:57
Watson BJ, Haitao Z, Atkinson GL, Young HM, Steven WH (2009) Processive Endoglucanases Mediate Degradation of Cellulose by Saccharophagus degradans. J Bacteriol 191(18):5697–5705
Wei KSC, Teoh TC, Koshy P, Salmah I, Zainudin A (2015) Cloning, expression and characterization of the endoglucanase gene from Bacillus subtilis UMC7 isolated from the gut of the indigenous termite Macrotermes malaccensis in Escherichia coli. Electro J Biotechnol 18:103–109
Wolfgang DE, Wilson DB (1999) Mechanistic studies of active site mutants of Thermomonospora fusca endocellulase E2. Biochemistry 38:9746–9751
Wonganu B, Pootanakit K, Booyapakron K, Champreda V, Tanaponqpipat S, Eurwilaichitr L (2008) Cloning, expression and characterization of a thermotolerant endoglucanase from Syncephalastrum racemosum (BCC18080) in Pichia pastoris. Protein Expr Purif 58:78–86
Yennamalli RM, Rader AJ, Kenny AJ, Wolt JD, Sen TZ (2013) Endoglucanases: insights into thermostability for biofuel applications. Biotechnol Biofuels 6:136
Zafar M, Ahmed S, Khan MI, Jamil A (2014) Recombinant expression and characterization of a novel endoglucanase from Bacillus subtilis in Escherichia coli. Mol Biol Rep 41:3295–3302
Zhang X-Z, Sathitsuksanoh N, Zhang Y-HP (2010) Glycoside hydrolase family 9 processive endoglucanase from Clostridium phytofermentans: Heterologous expression, characterization, and synergy with family 48 cellobiohydrolase. Bioresour Technol 101:5534–5538
Zheng B, Yang W, Wang Y, Feng Y, Lou Z (2009) Crystallization and preliminary crystallographic analysis of thermophilic cellulase from Fervidobacterium nodosum Rt17-B1. Acta Crystallogr Sect F Struct Biol Cryst Commun 65:219–222
Zhou WL, Irwin DC, Escovar-Kousen J, Wilson DB (2004) Kinetic studies of Thermobifida fusca Cel9A active site mutant enzymes. Biochem 43(30):9655–9663
Acknowledgments
This work was supported by a Grant No. 27(54)/2007-DSA (P&D) from the Ministry of Science and Technology, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests. We assure the quality and integrity of our research work. This study is completely independent and impartial, all points taken from other authors are well cited in the text. The research work was financially funded by Ministry of Science and Technology, Pakistan.
Additional information
Ikram ul Haq and Fatima Akram have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Haq, I.u., Akram, F., Khan, M.A. et al. CenC, a multidomain thermostable GH9 processive endoglucanase from Clostridium thermocellum: cloning, characterization and saccharification studies. World J Microbiol Biotechnol 31, 1699–1710 (2015). https://doi.org/10.1007/s11274-015-1920-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1920-4