Abstract
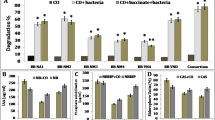
Soil contaminated by hydrocarbons, cannot be used for agricultural intents due to their toxic effect to the plants. Surfactants producing by plant growth promotory rhizobacteria (PGPR) can effectively rig the problem of petroleum hydrocarbon contamination and growth promotion on such contaminated soils. In the present study three Pseudomonas strains isolated from contaminated soil identified by 16S rRNA analysis were ascertained for PGPR as well as biosurfactants property. Biosurfactants produced by the strains were further characterized and essayed for rhamnolipids. Inoculation of the strains in petrol hydrocarbon contaminated soil and its interaction with Withania somnifera in presence of petrol oil hydrocarbons depict that the strains helped in growth promotion of Withania somnifera in petrol oil contaminated soil while rhamnolipids helped in lowering the toxicity of petrol oil. The study was found to be beneficial as the growth and antioxidant activity of Withania sominfera was enhanced. Hence the present study signifies that rhamnolipids producing PGPR strains could be a better measure for reclamation of petrol contaminated sites for growing medicinal plants.

Similar content being viewed by others
References
Abouseoud M, Maachi R, Amrane A (2008) Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination 223:143–151
Ahammed GJ, Wang MM, Zhou YH (2012) The growth, photosynthesis and antioxidant defense responses of five vegetable crops to phenanthrene stress. Ecotoxicol Environ Saf 80:132–139
Ali M, Shuaib M, Ansari SH (1997) Withanolides from the stem bark of Withania somnifera. Phytochemistry 44(6):1163–1168
Bashan Y, de Bashan LE (2005) Fresh-weight measurements of roots provide inaccurate estimates of the effects of plant growth-promoting bacteria on root growth: a critical examination. Soil Biol Biochem 37:1795–1804
Bashan Y, Kamnev AA, de Bashan LE (2013a) Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. Biol Fertil Soils 49:465–479
Bashan Y, Kamnev AA, de Bashan LE (2013b) A proposal for isolating and testing phosphate-solubilizing bacteria that enhance plant growth. Biol Fertil Soils 49:1–2
Bona C, Rezende I, Santos G, Souza L (2011) Effect of soil contaminated by diesel oil on the germination of seeds and the growth of Schinus terebinthifoilus. Brazilian archiv Biol Technol 54(6):1379–1387
Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I (2001) Antioxidant principles from Bauhinia tarapotensis. J Nat Prod 64(7):892–895
Cooper DG, Goldenberg BG (1987) Surface-active agents from two Bacillus species. Appl Environ Microbiol 53:224–229
Decker EA, Welch B (1990) Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem 38:674–677
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61(1):47–64
Dhanani T, Shah S, Gajbhiye NA, Kumar S (2013) Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arabian J Chem. doi:10.1016/j.arabjc.2013.02.015
Dibble JT, Bartha R (1979) Effect of environmental parameters on the biodegradation of oil sludge. Appl Environ Microbiol 37(4):729–739
Geys R, Soetaert W, Bogaert IV (2014) Biotechnological opportunities in biosurfactant production. Curr Opin Biotechnol 30:66–72
Glick BR (1995) The enhancement of plant growth by free living bacteria. Can J Microbiol 41:109–117
Graj W, Lisiecki P, Szulc A (2013) Bioaugmentation with petroleum-degrading consortia has a selective growth-promoting impact on crop plants germinated in diesel oil-contaminated soil. Water Air Soil Pollut 224:1676
Greaves JE (1922) Influence of salts on bacterial activities of soil. Bot Gaz 73:161–180
Hou FS, Milke MW, Leung DW, MacPherson DJ (2001) Variation in phytoremediation performance with diesel contaminated soil. Environ Technol 22:215–222
Imsande J (1998) Iron, sulfur and chlorophyll deficiencies: A need for an integrative approach in plant physiology. Physiol Plant 103:139–144
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India Pvt. Ltd., New Delhi
Khopade A, Ren B, Liu XY, Mahadik K, Zhang L, Kokare C (2012) Production and characterization of biosurfactant from marine Streptomyces species B3. J Colloid Interface Sci 367:311–318
Kloepper JW, Schroth MN (1978) Plant growth-promoting rhizobacteria on radishes: proceedings of the 4th internatational conference on plant pathogenic bacteria, Station de Pathologie Vegetable et Phytobacteriologie, INRA, Angers, France, 879–882
Kuiper I, Lagendijk EL, Bloemberg GV, Lugtenberg BJJ (2004) Rhizoremediation: a beneficial plant-microbe interaction. Mol Plant Microbe Interact 17:6–15
Kulakow PA, Schwab AP, Banks MK (2000) Screening plant species for growth on weathered, petroleum hydrocarbon-contaminated sediments. Int J Phytorem 2:297
Lai CC, Huang YC, Wei YH, Chang JS (2009) Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J Hazard Mater 167(1–3):609–614
Loper JE, Scroth MN (1986) Influence of bacterial sources on indole-3 acetic acid on root elongation of sugarbeet. Phytopathol 76:386–389
Martia MC, Camejoa D, Garcia NV (2009) Effect of oil refinery sludges on the growth and antioxidant system of alfalfa plants. J Hazard Mater 171:879–885
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilising microorganisms. FEMS Microbiol Lett 170:265–270
Oyaizu M (1986) Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:755–762
Pothuluri JV, Cerniglia CE (1994) Microbial metabolism of polycyclic aromatic hydrocarbons. In: Chaudhary RG (ed) Biological degradation and bioremediation of toxic chemicals. Chapman & Hall, London, pp 92–124
Ron EZ, Rosenberg E (2002) Biosurfactants and oil bioremediation. Curr Opin Chem Biol 13:249–252
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Siegmund I, Wagner F (1991) New method for detecting rhamnolipids excreted by Pseudomonas species during growth on mineral agar. Biotechnol Tech 5:265–268
Tahzibi A, Kamal F, Assadi MM (2004) Improved production of rhamnolipds by a Pseudomonas aeruginosa mutant. Iran Biomed J 8:25–31
Urum K, Pekdemir T (2004) Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere 57(9):1139–1150
Urum K, Grigson S, Pekdemir T, McMenamy S (2006) A comparison of the efficiency of different surfactants for removal of crude oil from contaminated soils. Chemosphere 62(9):1403–1410
Wang J, Zhang ZZ, Su YM, He W, He F, Song HG (2008) Phytoremediation of petroleum polluted soil. Petrol Sci 5(2):167
Yang J, Kloepper JW, Ryu CM (2008) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14(1):1–4
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Das, A.J. & Juwarkar, A.A. Reclamation of petrol oil contaminated soil by rhamnolipids producing PGPR strains for growing Withania somnifera a medicinal shrub. World J Microbiol Biotechnol 31, 307–313 (2015). https://doi.org/10.1007/s11274-014-1782-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-014-1782-1