Abstract
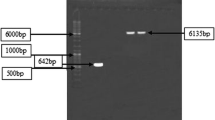
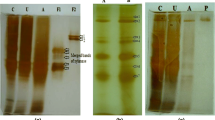
A xylanase producer, Bacillus pumilus SB-M13, was isolated from soil and identified using various tests based on carbohydrate fermentation preferences and fatty acid analysis. Xylanase gene, isolated using PCR amplification, was partially sequenced and it showed 89–94% sequence similarity to the xylanase genes of other B. pumilus strains. Xylanase with very low level of cellulase was produced on agricultural byproducts. The enzyme has been purified 186-fold by hydrophobic interaction chromatography and biochemically characterized. It has a molecular weight of 24.8 kDa and pI of 9.2. Xylanolytic activity is stable at alkaline pH and highest activity is observed at 60 °C and pH 7.5. Enzyme K m and k cat values were determined as 1.9 mg/mL and 42,600 U/mg, respectively. In aqueous-two-phase system, xylanase always partitioned to the top phase. Basic pH, low PEG concentration, salt addition, and presence of microbial cells enhanced xylanase partitioning. A maximum sevenfold purification, 10-fold concentration and 100% xylanase recovery were obtained, separately, by adjusting system parameters. A fourfold concentrated xylanase was obtained with 70% enzyme recovery only in one step ATPS process without cell harvesting.






Similar content being viewed by others
References
Adney A, Baker J (1996) Standard biomass analytical methods: chemical analysis and testing task laboratory analytical procedure LAP-006: measurement of cellulase activities, National Renewable Energy Laboratory, Midwest Research Institute
Albertsson PA (1986) Partitioning of cell particles and macromolecules, 3rd edn. Wiley, New York
Avcioglu B, Eyupoglu B, Bakir U (2005) Production and characterization of xylanases of a Bacillus strain isolated from soil. World J Microbiol Biotechnol 21:65–68
Bailey MJ, Biely P, Pountanen K (1992) Interlaboratory testing methods for xylanase activity. J Biotechnol 23:257–270
Bakir U (2004) Encyclopedia of bioresource technology: microbial xylanase production. Haworth Press, Ashok Pandey, pp 592–600
Bakir U, Yavascaoglu S, Guvenc F, Ersayin A (2001) An endo-β-1, 4-xylanase from Rhizopus oryzae: production partial purification and biochemical characterization. Enzyme Microb Technol 29:328–334
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microb Biotechnol 56:326–338
Belancic A, Scarpa J, Peirano A, Diaz R, Steiner J, Eyzayuirre J (1995) Penicillium purpurogenum produces several xylanases: purification and properties of two of the enzymes. Biotechnol J 41:71–79
Betini JHA, Michelin M, Peixoto-Nogueira SC, Jorge JA, Terenzi HF, Polizeli MLTM (2009) Xylanases from Aspergillus niger, Aspergillus niveus and Aspergillus ochraceus produced under solid-state fermentation and their application in cellulose pulp bleaching. Bioprocess Biosyst Eng 32:819–824
Bim MA, Franco TT (2000) Extraction in aqueous two-phase systems of alkaline xylanase produced by Bacillus pumilus and its application in kraft pulp bleaching. J Chromatogr B 743:349–356
Blum H, Beier H, Grass HJ (1987) Improved silver staining of plant proteins RNA and DNA in polyacrylamide gels. Electrop 8:93–99
Bradford MM (1976) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Anal Biochem 72:248–254
Collins T, Gerday C, Feller G (2005) Xylanases xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Costa SA, Pessoa A, Roberto IC (1998) Xylanase recovery- effect of extraction conditions on the aqueous two-phase system using experimental design. Appl Biochem Biotechnol 70:629–639
Cui F, Li Y, Li Y, Liu Z, Zhao H, Ping L, Ping L, Yang Y, Xue Y, Yan L (2009) Optimization of fermentation conditions for production of xylanase by a newly isolated strain, Penicillium thiersii ZH-19. World J Microbiol Biotechnol 25:721–725
de Buyl E, Lahaye A, Ledoux P, Amory A, Detroz R, Andre C, Vetter R (1998) United States Patent, Patent no: 6,426,211
Duarte MCT, Portugal EP, Ponezi AN, Bim MA, Tagilari CV, Franco TT (1999) Production and purification of alkaline xylanases. Bioresource Technol 68:49–53
Duarte MCT, Pellegrino ACA, Portugal EP, Ponezi AN, Franco TT (2000) Characterization of alkaline xylanases from Bacillus pumilus. Brazillian J Microbiol 31:90–94
Gomes I, Gomes J, Steiner W, Esterbauer H (1992) Production of cellulase and xylanase by a wild strain of Trichoderma viride. Appl Microbiol Biotechnol 36:701–707
Jain A, Johri BN (1999) Partitioning of an extracellular xylanase produced by a thermophilic fungus Melanocarpus albomyces IIS-68 in an aqueous two-phase system. Bioresource Technol 67:205–207
Johansson G (1986) Partitioning in aqueous two-phase systems: theory, methods, uses and applications to biotechnology. Academic Press, London
Khoodoo MHR, Sahin F, Donmez MF, Fakim YJ (2005) Molecular characterisation of Xanthomonas strains isolated from aroids in Mauritius Syst. Appl Microbiol 28:366–380
Kiddinamoorthy J, Anceno AJ, Haki GD, Rakshit SK (2008) Production, purification and characterization of Bacillus sp.GRE7 xylanase and its application in eucalyptus Kraft pulp biobleaching. World J Microbiol Biotechnol 24:605–612
Kulkarni N, Vaidya A, Rao M (1999) Extractive cultivation of recombinant Escherichia coli using aqueous two-phase systems for production and separation of extracellular xylanase Biochem. Biophy Res Comm 255:274–278
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head. Nature 227:680–685
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Okada H, Shinmyo A (1988) Xylanase of Bacillus pumilus. Methods Enzymol 160:632–638
Pham PL, Taillandier P, Delmas M, Strehaiano P (1998) Production of xylanases by Bacillus polymyxa using lignocellulosic wastes. Indust Crops Products 7:195–203
Posprech A, Neumann B (1995) A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet 11:217–218
Saleem M, Tabassum MR, Yasmin R, Imran M (2009) Potential of xylanase from thermophilic Bacillus sp. XTR-10 in biobleaching of wood kraft pulp. Int Biodeterioration Biodegradation 63:1119–1124
Sanger F (1977) Nucleotide sequence of bacteriophage phi X174 DNA. Nature 265:687–695
Sasser MJ (1990) Identification of bacteria by gas chromatography of cellular fatty acids, technical note 101. Microbial ID, Newark
Subramaniyan S, Prema P (2000) Cellulase-free xylanases from Bacillus and other microorganisms. FEMS Microbiol Lett 183:1–7
Wang P, Mason JC, Broda P (1993) Xylanases from Streptomyces cyaneus: their production, purification and characterization. J Gen Microbiol 139:1987–1993
Wong KKY, Saddler JN (1993) Hemicellulose and hemicellulases: applications of hemicellulases in the food, feed and pulp and paper industries. Portland Press, London, pp 127–143
Yang VW, Zhuang Z, Elegir G, Jeffries TW (1995) Alkaline-active xylanase produced by an alkaliphilic Bacillus sp. isolated from kraft pulp. J Ind Microbiol 15:434–441
Acknowledgments
This project was supported by TÜBİTAK (MISAG-177, and MISAG-104T473), Middle East Technical University (AFP-99-06-02-10) and BAP-0811-DPT2002K120510-BTEK08. The authors offer sincere thanks to TUBITAK, METU and DPT. We would like to express our gratitude to staff of METU Molecular Biology-Biotechnology R&D Center, who has always offered instant and high quality assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ersayin Yasinok, A., Biran, S., Kocabas, A. et al. Xylanase from a soil isolate, Bacillus pumilus: gene isolation, enzyme production, purification, characterization and one-step separation by aqueous-two-phase system. World J Microbiol Biotechnol 26, 1641–1652 (2010). https://doi.org/10.1007/s11274-010-0340-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0340-8