Abstract
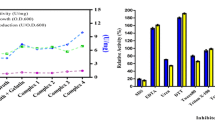
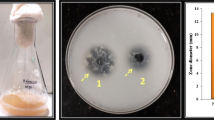
An extremely halophilic Chromohalobacter sp. TVSP101 was isolated from solar salterns and screened for the production of extracellular halothermophilic protease. Identification of the bacterium was done based upon biochemical tests and the 16S rRNA sequence. The partially purified enzyme displayed maximum activity at pH 8 and required 4.5 M of NaCl for optimum proteolytic activity. In addition, this enzyme was thermophilic and active in broad range of temperature 60–80°C with 80°C as optimum. The Chromohalobacter sp. required 4 M NaCl for its optimum growth and protease secretion and no growth was observed below 1 M of NaCl. The initial pH of the medium for growth and enzyme production was in the range 7.0–8.0 with optimum at pH 7.2. Various cations at 1 mM concentration in the growth medium had no significant effect in enhancing the growth and enzyme production but 0.5 M MgCl2 concentration enhanced enzyme production. Casein or skim milk powder 1% (w/v) along with 1% peptone proved to be the best nitrogen sources for maximum biomass and enzyme production. The carbon sources glucose and glycerol repressed the protease secretion. Immobilization of whole cells in absence of NaCl proved to be useful for continuous production of halophilic protease.






Similar content being viewed by others
References
Arahal DR, Garcia MT, Ludwig W, Schleifer KH, Ventosa A (2001a) Transfer of Halomonas canadensis and Halomonas israelensis to the genus Chromohalobacter as Chromohalobacter canadensis comb. nov. and Chromohalobacter israelensis comb. nov. Int J Syst Evol Microbiol 51:1443–1448
Arahal DR, Garcia MT, Vargas C, Canovas D, Nieto JJ, Ventosa A (2001b) Chromohalobacter salexigens sp. nov., a moderately halophilic species that includes Halomonas elongate DSM 3043 and ATCC33174. Int J Syst Evol Microbiol 51:1457–1462
Bagai R, Madamwar D (1997) Continuous production of halophilic α-amylase through whole cell immobilization of Halobacterium salinarium. Appl Biochem Biotechnol 62:213–218
Beddow CG, Ardeshir AG (1979) The production of soluble fish protein solution for use in fish sauce manufacture. I. The use of added enzymes. J Food Technol 14:603–612
Bonete MJ, Pire C, Llorca FI, Camacho ML (1996) Glucose dehydrogenase from the Halophilic archaeon Haloferax mediterranei: enzyme purification, characterization and N–terminal sequence. FEBS Lett 383:227–229
Brock FM, Frosberg CW, Buchanan-Smith JG (1982) Proteolytic activity of rumen microorganisms and effect of proteinase inhibitors. Appl Environ Microbiol 44:561–569
Cadenas Q, Engel PC (1994) Activity staining of halophilic enzymes: substitution of salt with a zwitterions in non-denaturing electrophoresis. Biochem Mol Biol Int 33:785–792
Danson MJ, Hough DW (1997) The structural basis of protein halophilicity. Comp Biochem Physiol 117:307–312
Drucker H (1972) Regulation of exocellular proteases in Neurospora crassa: induction and repression of enzyme synthesis. J Bacteriol 110:1041–1049
Ferrero MA, Castro GR, Abate CM, Baigori MD, Sineriz F (1996) Thermostable alkaline protease of Bacillus licheniformis MIR-29 isolation production and characterization. Appl Microbiol Biotechnol 45:327–332
Gildberge A (1989) Accelerated fish sauce fermentation by initial alkalification at low salt concentration. In Miyachi S, Karube I, Ishida Y (eds.), Current topics in marine biotechnology. Fuji Technol Press, Tokyo, pp101–104
Gomes J, Steiner W (2004) The biocatalytic potential of extremophiles and extremozymes. Food Technol Biotechnol 42:223–235
Izotova LS, Strongin AY, Chekulaeva LW, Sterkin VE, Ostoslavskaya VI, Lyublinskaya EA, Timokhina EA, Stepanov VM (1983) Purification and properties of serine protease from Halobacterium halobium. J Bacteriol 155:826–830
Joo HS, Kumar CG, Park GC, Paik SR, Chang CS (2003) Oxidant and SDS-stable alkaline protease from Bacillus clausii I-52: production and some properties. J Appl Microbiol 95:267–272
Kamekura M, Seno Y, Holmes ML, Dyall-Smith ML (1992) Molecular cloning and sequencing of the gene for a halophilic alkaline serine protease (halolysin) from an unidentified halophilic archaea strain (172P1) and expression of the gene in Haloferax volcanii. J Bacteriol 174:736–742
Kamekura M, Seno Y, Dyall-Smith ML (1996) Halolysin R4, a serine proteinase from the halophilic archaeon Haloferax mediterranei; gene cloning, expression and structural studies. Biochimica Biophysica Acta 1294:159–167
Kanlayakrit W, Preeyanuch B, Takuji O, Masatoshi G (2004) Production and characterization of protease from an extremely halophilic Halobacterium sp. PB407. Kasetsart J (Nat Sci) 38:15–20
Kim J, Dordick JS (1997) Unusual salt and solvent dependence of a protease from an extreme halophile. Biotechnol Bioeng 55:471–479
Lanyi JK (1974) Salt-dependant properties of proteins from extremely halophilic bacteria. Bacteriol Rev 38:272–290
Margesin R, Schiner F (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83
Oren A, Ventosa A, Grant WD (1997) Proposed minimal standards for description of new taxa in the order Halobacteriales. Int J Syst Bacteriol 47:233–238
Prado B, Lizama C, Aguilera M, Cormenzana AR, Fuentes S, Campos V, Monteoliva-Sanchez M (2006) Chromohalobacter nigrandesensis sp. nov., a moderately halophilic, Gram-negative bacterium isolated from lake Tebenquiche on the Atacama Saltern, Chile. Int J Syst Evol Microbiol 56:647–651
Quillaguaman J, Delgado O, Mattiasson B, Hatti-Kaul R (2004) Chromohalobacter sarecensis sp. nov., a psychrotolerant moderate halophile isolated from the saline Andean region of Bolivia. Int J Syst Evol Microbiol 54:1921–1926
Schiraldi C, Giuliano M, De Rosa M (2002) Perspectives on biotechnological applications of archaea. Archaea 1:75–86
Schmitt W, Rdest U, Goebel W (1990) Efficient high performance liquid chromatographic system for the purification of Halobacterial serine protease. J Chromatogr 521:211–220
Stepanov VM, Rudenskaya GN, Revina LP, Gryaznova YB, Lysogorskaya EN, Filippova IY, Ivanova I (1992) A serine proteinase of archae bacterium, Halobacterium mediterranei, A homologue of an eubacterial subtilisin. Biochem J 285:281–286
Thongthai C, Suntinanalert P (1991) Halophiles in Thai fish sauce (nam pla). In: Rodriguez-Valera F (ed), General and applied aspects of halophilic microorganisms. Plenum Press, NewYork, pp381–388
Van den Berg B (2003) Extremophiles as source for novel enzymes. Curr Opin Microbiol 6:213–218
Ventosa A, Nieto JJ, Oren A (1988) Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev 62:504–544
Ventosa A, Nieto JJ, Oren A (1995) Biotechnological applications and potentialities of halophilic microorganisms. World J Microbiol Biotechnol 11:85–94
Vidyasagar M, Prakash S, Sreeramulu K (2006) Optimization of culture conditions for the production of haloalkaliphilic thermostable protease from an extremely halophilic archaeon Halogeometricum sp. TSS101. Lett Appl Microbiol 43:385–391
Acknowledgements
K. Sreeramulu is thankful to the DST, New Delhi, India for the financial assistance and M. Vidyasagar is indebted to UGC-CSIR, New Delhi for JRF fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vidyasagar, M., Prakash, S., Jayalakshmi, S.K. et al. Optimization of culture conditions for the production of halothermophilic protease from halophilic bacterium Chromohalobacter sp. TVSP101. World J Microbiol Biotechnol 23, 655–662 (2007). https://doi.org/10.1007/s11274-006-9279-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-006-9279-1