Abstract
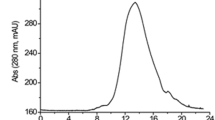
A novel fibrinolytic enzyme subtilisin FS33 was purified from Bacillus subtilis DC33, isolated from a traditional flavour-rich food in China. The purified subtilisin FS33 was a single chain protein with a molecular mass of 30 kDa measured by SDS-PAGE. After activated SDS-PAGE, the enzyme band exhibited strong fibrinolytic activity on the fibrin plate. Subtilisin FS33 was temperature-stable below 60°C over the pH range 5–12, with a maximum activity at pH 8.0, but the activity completely disappeared after 10 min above 65°C. The NH2-terminal amino acid sequence of the enzyme was different from that of other known fibrinolytic enzymes, such as NK, CK, SMCE, KA38, subtilisin E, subtilisin DFE and Katsuwokinase. The amidolytic activities of subtilisin FS33 were inhibited completely by phenylmethanesulfonyl fluoride (PMSF) and soybean trypsin inhibitor (SBTI). EDTA did not affect the enzyme activity, and none of the ions tested activated the activity. Therefore, the enzyme was thought to be a subtilisin-like serine protease. The enzyme degraded the Bβ-chains of fibrin(ogen) very rapidly and then degraded the Aα-chain and at least five fragments from fibrin(ogen) were obtained after hydrolysis. Subtilisin FS33 was also able to cleave blood clots in the absence of endogenous fibrinolytic factors.
Similar content being viewed by others
References
Arai K, Mimuro J, Madoiwa S, Matsuda M, Sako T, Sakata Y (1995) Effect of staphylokinase concentration of plasminogen activation. Biochim Biophys Acta 1245:69–75
Astrup T, MuEllertz S (1952) The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys 40:346–351
Bode C, Runge M, Smalling RW (1996) The future of thrombolysis in the treatment of acute myocardial infarction. Eur Heart J 17:55–60
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Fujita M, Nomura K, Hong K, Ito Y, Asada A (1993) Purification and characterization of a strong fibrinolytic enzyme (nattokinase) in the vegetable cheese natto, a popular soybean fermented food in Japan. Biochem Biophys Res Commun 197:1340–1347
Fujita M, Hong K, Ito Y, Fujii R, Kariya K, Nishimuro S (1995a) Thrombolytic effect of nattokinase on a chemically induced thrombosis model in rat. Biol Pharm Bull 18:1387–1391
Fujita M, Hong K, Ito Y (1995b) Transport of NK across of the rat intestinal tract. Biol Pharm Bull 18:1194–1196
Glazer AN (1967) Esteratic reactions catalysed by subtilisins. J␣Biol Chem 242:433–436
Kim JH, Kim YS (1999) A fibrinolytic metalloprotease from the fruiting bodies of an edible mushroom, Armillariella mellea. Biosci Biotechnol Biochem 63:2130–2136
Kim W, Choi K, Kim Y (1996) Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK11–4 screened from Chungkook-Jang. Appl Environ Microbiol 62:2482–2488
Kim HK, Kim GT, Kim DK, Choi WA (1997) Purification and characterization of a novel fibrinolytic enzyme from Bacillus sp. KA38 originated from fermented fish. J Fermen Bioeng 84:307–312
Krieg N, Holt JG (1984) Bergey’s manual of systematic bacteriology, vol 1, 9th edn. Williams and Wilkins, University of Tokyo press, pp 120–340. ISBN: 01985755x
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Medved LV, Solovjov DA, Ingham KC (1966) Domain structure, stability and interactions in streptokinase. Eur J Biochem 239:333–339
Moukhametova LI, Aisina RB, Lomakina GY, Varfolomeev SD (2002) Properties of the urokinase-type plasminogen activator modified with phenylglyoxal. Russ J Bioorg Chem 28:278–283
Peng Y, Huang Q, Zhang RH, Zhang YZ (2003) Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4. Comp Biochem Physiol B 134:45–52
Peng Y, Yang XJ, Zhang YZ (2005) Microbial fibrinolytic enzymes: an overview of source, production, properties, and thrombolytic activity in vivo. Appl Microbiol Biotechnol 0175-7598, 1432–0614 (Online). DOI: 10.1007/s 00253-005-0159-7
Price N, Stevens L (1989) Fundamentals of enzymology, 2 edn. Oxford University Press, Oxford. ISBN: 019850229x
Sumi H, Hamada H, Tsushima H (1987) A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto, a typical and popular soybean food in the Japanese diet. Experientia 43:1110–1111
Sumi H, Hamada H, Nakanishi K, Hiratani H (1990) Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol 84:139–143
Sumi H, Yanagisawa Y, Yatagai C (2004) Natto bacillus as oral␣fibrinolytic agent: Nattokinase activity and the ingestion␣effect of Bacillus natto. Food Sci Technol Int 10(1):17–20
Takahashi M, Sekine T, Kuba N, Nakamori S, Yasuda M (2004) The production of recombinant APRP, an alkaline protease derived from Bacillus pumilus TYO-67, by in vitro refolding of pro-enzyme fixed on a solid surface. J Biochem 136:549–556
Wang CT, Ji BP, Li B, Ji H (2003) Isolation of Douchi fibrinolytic enzyme producing strain DC33 from Chinese Douchi and primary analysis of the enzyme property. Food Sci (China) 6:34–38
Wong AHK, Mine Y (2004) A novel fibrinolytic enzyme in fermented shrimp paste. A traditional Asian fermented seasoning. J Agric Food Chem 52:980–986
Wong SL, Price CW, Goldfarb DS, Doi RH (1984) The subtilisin E gene of Bacillus subtilis is transcribed from a sigma-37 promoter in vivo. Proc Nat Acad Sci USA 81:1184–1188
Acknowledgment
We thank Prof. Rob Nout (Department of Agrotechnology and Food Sciences, Wageningen University, The Netherlands) for assistance in revision of the manuscript and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Ji, B., Li, B. et al. Enzymatic properties and identification of a fibrinolytic serine protease purified from Bacillus subtilis DC33. World J Microbiol Biotechnol 22, 1365–1371 (2006). https://doi.org/10.1007/s11274-006-9184-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-006-9184-7