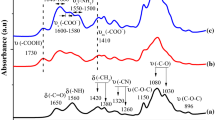
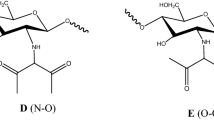
Summary
Chitosan is an amino-polysaccharide with highly efficient properties for the binding of metal ions and anionic dyes. Uptake may occur through chelation on free amino functions (at near-neutral pH) or by electrostatic attraction on protonated amino groups (in acidic solutions). The polymer is soluble in acidic solutions and its binding properties can be used in both solid form (sorption) and liquid form (ultrafiltration coupled with chelation, coagulation–flocculation). These properties have been used for the recovery of mercury from dilute solutions at initial pH 5 (which reveals the most efficient pH in the range pH 4–6) and for the recovery of Reactive Black 5 (RB5, anionic dye) at pH 3. While in the case of mercury binding saturation of the biopolymer is only slightly higher when chitosan is used in the liquid form compared to solid-state adsorption, in the case of the coagulation–flocculation of RB5 (using the liquid-form of chitosan) the saturation of the polymer (calculated on the basis of molar ratio of dye vs. amino groups of the polymer) is reached at a significantly greater value than when the polymer is used for the solid-state binding of the dye. There is a much more efficient use of amino groups when chitosan is used in the liquid-form due to a better availability of amino groups (less hydrogen bonds between the chains of the polymer) and to a better accessibility to internal sorption sites (lower diffusion control).
Similar content being viewed by others
References
G. Annadurai R.-S. Juang D.-J. Lee (2002) ArticleTitleUse of cellulose-based wastes for adsorption of dyes from aqueous solutions Journal of Hazardous Materials B92 263–274
J.P. Chen L. Wang (2001) ArticleTitleCharacterization of a ca-alginate based ion-exchange resin and its application in lead, copper, and zinc removal Separation Science and Technology 36 3617–3637 Occurrence Handle1:CAS:528:DC%2BD38XosF2jtg%3D%3D
M.-S. Chiou H.-Y. Li (2002) ArticleTitleEquilibrium and modeling of adsorption of reactive dye on cross-linked chitosan beads Journal of Hazardous Materials B93 233–248
L. Dambies T. Vincent A. Domard E. Guibal (2001) ArticleTitlePreparation of chitosan gel beads by ionotropic molybdate gelation Biomacromolecules 2 1198–1205 Occurrence Handle10.1021/bm010083r Occurrence Handle1:CAS:528:DC%2BD3MXntlens7Y%3D
M.A. Dubois J.F. Dozol C. Massiani (1995) ArticleTitlePyrolysis and incineration of cationic and anionic ion-exchange resins–identification of volatile degradation compounds Journal of Analytical and Applied Pyrolysis 31 129 Occurrence Handle10.1016/0165-2370(94)00817-K Occurrence Handle1:CAS:528:DyaK2MXlsFCls7c%3D
M.S. Dzul Erosa T.I. Saucedo Medina R. Navarro Mendoza M. Avila Rodriguez E. Guibal (2001) ArticleTitleCadmium sorption on chitosan sorbents: kinetic and equilibrium studies Hydrometallurgy 61 157–167 Occurrence Handle10.1016/S0304-386X(01)00166-9 Occurrence Handle1:CAS:528:DC%2BD3MXkvVWmtb8%3D
G. Gibbs J.M. Tobin E. Guibal (2003) ArticleTitleSorption of acid green 25 on chitosan: influence of experimental parameters on uptake kinetics and sorption isotherms Journal of Applied Polymer Science 90 1073–1080 Occurrence Handle10.1002/app.12761 Occurrence Handle1:CAS:528:DC%2BD3sXntFelsbk%3D
G. Gibbs J.M. Tobin E. Guibal (2004) ArticleTitleInfluence of chitosan pre-protonation on reactive black 5 sorption isotherms and kinetics Industrial and Engineering Chemical Research 43 1–11 Occurrence Handle1:CAS:528:DC%2BD3sXpsVaqurg%3D
E. Guibal (2004) ArticleTitleMetal ion interactions with chitosan–a review Separation and Purification Technology 38 43–74 Occurrence Handle10.1016/j.seppur.2003.10.004 Occurrence Handle1:CAS:528:DC%2BD2cXks1Khsr0%3D
C. Huang S. Chen J.R. Pan (2000) ArticleTitleOptimal condition for modification of chitosan: a biopolymer for coagulation of colloidal particles Water Research 34 1057–1062 Occurrence Handle1:CAS:528:DC%2BD3cXntVKnsg%3D%3D
C. Huang Y. Chen (1996) ArticleTitleCoagulation of colloidal particles in water by chitosan Journal of Chemical Technology and Biotechnology 66 227–232 Occurrence Handle10.1002/(SICI)1097-4660(199607)66:3<227::AID-JCTB499>3.0.CO;2-M Occurrence Handle1:CAS:528:DyaK28XktlCjs7k%3D
R.-S. Juang C.-H. Chiou (2000) ArticleTitleUltrafiltration rejection of dissolved ions using various weakly basic water-soluble polymers Journal of Membrane Science 177 207–214 Occurrence Handle10.1016/S0376-7388(00)00464-6 Occurrence Handle1:CAS:528:DC%2BD3cXlsFertLw%3D
R.-S. Juang R.-L. Tseng F.-C. Wu S.-H. Lee (1997) ArticleTitleAdsorption behavior of reactive dyes from aqueous solutions on chitosan Journal of Chemical Technology and Biotechnology 70 391–399 Occurrence Handle10.1002/(SICI)1097-4660(199712)70:4<391::AID-JCTB792>3.0.CO;2-V Occurrence Handle1:CAS:528:DyaK1cXhtlaqtw%3D%3D
Y. Kawamura M. Mitsushashi H. Tanibe H. Yoshida (1993) ArticleTitleAdsorption of metal ions on polyaminated highly porous chitosan chelating resin Industrial and Engineering Chemical Research 32 386–391 Occurrence Handle1:CAS:528:DyaK3sXmtFynug%3D%3D
B. Krajewska (2001) ArticleTitleDiffusion of metal ions through gel chitosan membranes Reactive and Functional Polymers 47 37–47 Occurrence Handle10.1016/S1381-5148(00)00068-7 Occurrence Handle1:CAS:528:DC%2BD3MXhslKhu7w%3D
Kuncoro, E.K., Roussy, J. & Guibal, E. 2004 Mercury recovery by polymer-enhanced ultrafiltration: comparison of chitosan and poly(ethyleneimine) used as macro-ligand. Separation Science and Technology in press,.
P. McCarrick J. Tobin E. Guibal (2003) ArticleTitleComparative sorption of dyes on chitosan and activated carbon Separation Science and Technology 38 3049–3073
G. McKay H.S. Blair J.R. Gardner (1987) ArticleTitleTwo resistance mass transport model for the adsorption of acid dye onto chitin in fixed beds Journal of Applied Polymer Science 33 1249–1257 Occurrence Handle10.1002/app.1987.070330416 Occurrence Handle1:CAS:528:DyaL2sXkvFOjsLY%3D
F.-L. Mi S.-S. Shyu S.-T. Lee T.-B. Wong (1999) ArticleTitleKinetic study of chitosan-tripolyphosphate complex reaction and acid-resistive properties of the chitosan-tripolyphosphate gel beads prepared by in-liquid curing method Journal of Polymer Science Part B. Polymer Physics 37 1551–1564 Occurrence Handle10.1002/(SICI)1099-0488(19990715)37:14<1551::AID-POLB1>3.0.CO;2-H Occurrence Handle1:CAS:528:DyaK1MXkt1Sgtb8%3D
J.R. Pan C. Huang S. Chen Y.-C. Chung (1999) ArticleTitleEvaluation of a modified chitosan biopolymer for coagulation of colloidal particles Colloids and Surfaces A: Physicochemical and Engineering Aspects 147 359–364 Occurrence Handle1:CAS:528:DyaK1MXps1OhtQ%3D%3D
E. Piron M. Accominotti A. Domard (1997) ArticleTitleInteraction between chitosan and uranyl ions. Role of physical and physicochemical parameters on the kinetics of sorption Langmuir 13 1653–1658 Occurrence Handle10.1021/la960765d Occurrence Handle1:CAS:528:DyaK2sXisF2msr4%3D
J. Roussy M. Vooren ParticleVan E. Guibal (2004) ArticleTitleChitosan for the coagulation and flocculation of mineral colloids Dispersion Science and Technology 25 663–677 Occurrence Handle1:CAS:528:DC%2BD2cXnvVersbo%3D
M. Ruiz A.M. Sastre E. Guibal (2000) ArticleTitlePalladium sorption on glutaraldehyde-crosslinked chitosan Reactive and Functional Polymers 45 155–173 Occurrence Handle10.1016/S1381-5148(00)00019-5 Occurrence Handle1:CAS:528:DC%2BD3cXnslWlsLs%3D
P. Sorlier A. Denuzière C. Viton A. Domard (2001) ArticleTitleRelation between the degree of acetylation and the electrostatic properties of chitin and chitosan Biomacromolecules 2 765–772 Occurrence Handle10.1021/bm015531+ Occurrence Handle1:CAS:528:DC%2BD3MXkvFCrur4%3D
Y.C. Wong Y.S. Szeto W.H. Cheung G. McKay (2004) ArticleTitleAdsorption of acid dyes on chitosan-equilibrium isotherm analyses Process Biochemistry 39 695–704 Occurrence Handle10.1016/S0032-9592(03)00152-3
H. Yoshida T. Takemori (1997) ArticleTitleAdsorption of direct dye on cross-linked chitosan fiber: breakthrough curve Water Science and Technology 35 29–37 Occurrence Handle10.1016/S0273-1223(97)00111-X Occurrence Handle1:CAS:528:DyaK2sXks1Slsbc%3D
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guibal, E., Touraud, E. & Roussy, J. Chitosan Interactions with Metal Ions and Dyes: Dissolved-state vs. Solid-state Application. World J Microbiol Biotechnol 21, 913–920 (2005). https://doi.org/10.1007/s11274-004-6559-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11274-004-6559-5