Abstract
Sphagnum cultivation is a type of paludiculture and a way to use formerly drained peatlands productively but under wet and therefore climate-friendly conditions. Where Sphagnum mosses are cultivated other plant species will also establish and possibly compete with the Sphagnum. The aim of this study was to determine which factors influence vascular plant cover as well as plant species numbers at Sphagnum cultivation sites and to derive recommendations for their management. Two cultivation sites were studied in northwest Germany. One of these was established directly after peat extraction while the other was rewetted seven years prior to establishment. Irrigation ditches for water management were installed at both sites. The cover of vascular plants and the number of plant species present were determined in systematically positioned plots. Six variables were tested for their influence on the assessed data by applying boosted regression tree models. The main factors influencing vascular plant cover at the two Sphagnum cultivation sites were the distance to an irrigation ditch (m), the site (location) and Sphagnum cover (%). The number of species per plot was influenced mainly by Sphagnum cover (%), the distance to an irrigation ditch (m) and the donor species used for initiating the cultivation sites. A sufficient supply of nutrient-poor water and optimal Sphagnum growth can reduce vascular plant cover and the number of plant species potentially present at a site. Insufficient water distribution and uneven Sphagnum establishment lead to inhomogeneous site conditions and thus to a higher number of plant species. The number and cover of plant species at a cultivation site are influenced by the vegetation of the sites’ surroundings and the selection of the donor site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biomass of Sphagnum mosses can be used as a renewable alternative for fossil peat in horticultural growing media because they have nearly identical physical and chemical properties (Emmel 2008; Oberpaur et al. 2010; Jobin et al. 2014; Kumar 2017; Müller and Glatzel 2021). In addition, living Sphagnum biomass can be introduced as donor material to rewetted peatlands for restoration (Quinty and Rochefort 2003). This aims to accelerate the development of characteristic Sphagnum-dominated vegetation and thus facilitate peat-formation (Campeau and Rochefort 1996; Robroek et al. 2009; González and Rochefort 2014; Karofeld et al. 2016, 2017; Hugron et al. 2020). For either case, Sphagnum biomass should not be collected on a large scale from natural peatlands, because they are very important carbon sinks and stores (Gorham 1991; Harenda et al. 2018; Beaulne et al. 2021; Loisel et al. 2021). They also provide important habitats for specialised flora and fauna (Rydin and Jeglum 2006; Joosten et al. 2017). An exception for collection in natural peatlands may be, when they are designated for peat extraction in countries with abundant peatlands (Quinty and Rochefort 2003). In other countries, Sphagnum-rich peatlands might not be available for collecting Sphagnum biomass at all because they have been destroyed by agricultural use, forestry or peat extraction (e.g. Germany, Netherlands). They are often strictly protected (Joosten 2012) or even regulated by the EU Habitats Directive (92/43/EEC).
New approaches are therefore needed to obtain Sphagnum biomass. One way to establish a renewable source for Sphagnum biomass is the establishment of Sphagnum cultivation sites (also known as Sphagnum farming) where Sphagnum mosses are propagated (Money 1994; Pouliot et al. 2015; Gaudig et al. 2018; Hugron and Rochefort 2018). These Sphagnum cultivation sites can be established on rewetted former bog grassland (Gaudig et al. 2014) or cut-over peatland (Pouliot et al. 2015; Gaudig et al. 2017; Grobe et al. 2021). The cultivation of Sphagnum on rewetted peatlands is one type of paludiculture, which is defined as a sustainable, productive use of peatlands under wet and thus peat-preserving conditions (Wichtmann et al. 2016). The raising of the water table required for this use can reduce high greenhouse gas emissions (Beyer and Höper 2015; Günther et al. 2017; Oestmann et al. 2021) that occur in conventional drainage-based peatland agriculture (Waddington and Price 2000; Tiemeyer et al. 2020).
To establish a cultivation site for Sphagnum it is necessary to first create an even, bare peat surface crossed by ditches for irrigation. If a degraded, nutrient-rich topsoil is present, such as on agriculturally used peatlands, it must be removed (Gaudig et al. 2018). Fragments of living Sphagnum mosses are then spread on the bare peat surface and covered with a protective material (most often straw) to protect the Sphagnum fragments from desiccation in the initial phase. As the final step the sites are rewetted. This procedure is similar to the “moss layer transfer technique” (MLTT) which is used for the restoration of peatlands by Sphagnum biomass introduction (Quinty and Rochefort 2003).
After the installation of a cultivation site, a closed Sphagnum carpet can develop which can be harvested every 3 to 5 years (Gaudig et al. 2014; Krebs et al. 2018). For optimal growth of the Sphagnum a stable, near-surface water table throughout the year is essential (Hayward and Clymo 1983; Pouliot et al. 2015; Gaudig et al. 2020). Besides the water table several other factors influence the establishment of Sphagnum when a cultivation site is being installed. These can be the layer thickness of the Sphagnum fragments or the type of protective cover used to protect the Sphagnum from desiccation in the initial phase as well as the distance of the growing point to the nearest irrigation ditch, which can influence the water availability (Gaudig et al. 2017; Grobe et al. 2021). Other factors such as the type and thickness of the residual peat layer, site management or the adjacent land use have been determined to influence the establishment of vegetation at restoration sites (Girard et al. 2002; Smolders et al. 2003; Laine et al. 2007; Triisberg et al. 2013; González and Rochefort 2014; Konvalinková and Prach 2014).
Similar factors might also influence the establishment of other plant species at cultivation sites. Those species are often also transferred, when living Sphagnum biomass is introduced from donor sites (González and Rochefort 2014; Karofeld et al. 2016; Hugron et al. 2020). In addition, species from the surrounding area are likely to migrate to the cultivation sites and establish there as well. Thus, the presence of plant species besides Sphagnum cannot be prevented entirely in in-situ cultivation. Especially vascular plants can become competition for the cultivated Sphagnum mosses, when their cover intercepts more than 50% of the photosynthetically active radiation (PAR) (Hayward and Clymo 1983). Vascular plants can also improve the growing conditions of Sphagnum mosses by increasing relative humidity, balancing surface temperatures or providing mechanical support (Malmer et al. 1994; Tuittila et al. 2000; Pouliot et al. 2011). However, if the cultivated Sphagnum fibres are to be used as a constituent for horticultural growing media, they must be as clean and free of other fibres as possible (Kumar 2017). On the other hand, if the aim is to produce donor material for restoration, the presence of other plant species may be tolerable, as long as they do not impede the growth of the target Sphagnum species, or even desirable if they are typical bog species. Plant species establishing in addition to the Sphagnum target species increase species diversity at cultivation sites, which thus provide substitute habitats for bog typical or threatened species.
Regardless of whether or not a cover of vascular plants or the establishment of other plant species are tolerated at cultivation sites, it is necessary to determine the factors influencing the cover and establishment of plant species besides Sphagnum (vascular plants, other bryophytes). Knowing these factors would allow to derive recommendations for their management at cultivation sites.
Following these considerations, the aim of this study was
-
to determine which factors influence vascular plant cover and plant species numbers at Sphagnum cultivation sites and.
-
to derive recommendations for the management of vascular plants and plant species numbers at cultivation sites.
Methods
Study sites
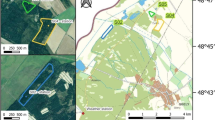
Two Sphagnum cultivation sites were established as a practical trial on cut-over peatland near Twist in northwest Germany (Fig. 1a), called “Drenth” (DRT) (52° 41′ N, 07° 05′ E) and “Provinzialmoor” (PRM) (52° 40′ N, 07° 06′ E). This scientific study was built upon the existing trial. The local climate is oceanic with a mean annual temperature of 10 °C and a mean annual precipitation of 800 mm (1981–2010, meteorological station Lingen) (DWD 2022). At both sites peat had been extracted using the “milled peat” method. After peat extraction, an average peat layer thickness of 61 cm at DRT and 95 cm at PRM remained above the mineral soil. The residual peat layer contained highly decomposed black peat with an average degree of humification after von Post (1924) of H7–H8.
After peat extraction terminated, polders separated by peat bunds were created at both sites. The drainage ditches were filled with peat and the area within the polders was levelled but not stripped. This is a common approach to preparing peatlands for rewetting after peat extraction in Germany (Blankenburg 2004). The cultivation site PRM was established within a rewetted polder and had a rectangular shape (60 × 160 m, 1 ha study site) (Fig. 1b). PRM was surrounded by 100 ha of rewetted cut-over peatland. The cultivation site DRT, on the other hand, was a narrow strip divided into three polders of 0.4 ha (25 × 160 m) each (total study site 1.2 ha) (Fig. 1c). DRT was surrounded by about 50 ha of active and therefore drained peat extraction area. After construction of the polders they were rewetted through shallow inundation by excess rainfall in winter. While at PRM rewetting was implemented seven years prior to the establishment of the cultivation site in 2008, DRT was rewetted concurrently with the establishment of the cultivation site in 2015. When the establishment of both cultivation sites started in 2015, irrigation ditches were dug in the polders with an excavator (DRT) and by hand or small trenching machine (PRM). These were 0.3 m deep and 0.3–0.7 m wide. Because PRM had been inundated in the previous seven years, the water table had to be lowered first. Then, the already present sparse vegetation was removed. The irrigation ditches were installed at PRM with a distance of 40–60 m. At DRT the ditches were dug every 10 m, connecting to a perimeter ditch on both sides with a distance of about 23 m. The irrigation ditches allowed the active control of the water table at the cultivation sites. At PRM, water inflow was controlled manually with an elbow pipe (KG pipe) that connected the surrounding polders, which were inundated with precipitation water, with the cultivation site. When these no longer carried sufficient water, water was pumped into the irrigation ditches from an adjacent ditch containing drainage water from areas with ongoing peat extraction. At DRT, excess precipitation during the winter months was stored in two retention basins with a total volume of 6.000 m3. The water was pumped into the irrigation ditches during the summer months using an electrical pump. The retention basins were supplemented with groundwater when the collected precipitation water ran low, using another electrical pump. An overflow was installed at both sites to prevent flooding.
Despite the efforts to maintain the water table close to the surface, fluctuations were high at both sites and relatively stable only in the winter months. At PRM the water table fluctuated between 13 above and 80 cm below peat surface (April 2016 to November 2018). This can be explained mainly by the drying out of the irrigation polders in the summer months and thus insufficient water supply. At DRT the fluctuations were slightly lower, between 15 cm above and 55 cm below peat surface (April 2016 to November 2018). This was due to the additional use of groundwater that was pumped into the irrigation ditches. The mean water table during the growing seasons (May to September) was 32 cm below peat surface at PRM and 11 cm below at DRT. The pH (mean ± SE) of the irrigation water was 4.2 ± 0.03 with an electrical conductivity of (mean ± SE) 93 ± 3 µS cm−1 at PRM. Due to the additional use of groundwater at DRT higher values of pH 5.5 ± 0.15 and electrical conductivity 152 ± 7 µS cm−1 have occurred (details regarding water table and quality in Grobe et al. 2021 and Oestmann et al. 2021).
At both cultivation sites, the aim was to cultivate two Sphagnum species (S. papillosum and S. palustre). Both species are in demand as donor material for restoration and have suitable fibres for horticultural growing media production (Emmel 2008). At DRT, the aim was to produce Sphagnum fibres for growing media and at PRM, donor material for restoration was cultivated. Sphagnum mosses were introduced to both cultivation sites using a manual adaptation of the “moss layer transfer technique” (MLTT). With this technique, fragments of Sphagnum mosses are collected at a donor site and spread onto the bare peat surface at a rewetted peatland site (Quinty and Rochefort 2003). Donor material of two Sphagnum species was collected manually at two near-natural mires at 60 and 200 km distances from the cultivation sites. In October 2015 (S. papillosum) and in March 2016 (S. palustre), respectively, fragments of 5–10 cm were collected at the donor sites. Directly after the collection, the donor material was spread manually (application density 60–80% cover) on the bare peat at the cultivation sites. Each species was spread in a separate section at both sites but in the same way and at the same time for comparability (Fig. 1). To protect the Sphagnum fragments from desiccation in the initial phase, two different types of protective cover were tested. Parts of the study sites were covered with straw mulch (750–800 kg ha−1, application density 80% cover) while other sections were covered with a geotextile (50% shade, made from UV-stable white polypropylene with a weight of 18 g m−2). At DRT, the geotextile was tested with both donor species while at PRM, it was only tested with S. papillosum. At both sites it was removed about six months after installation. To limit competition for the Sphagnum mosses, vascular plants at both sites were mown one to two times (spring, winter) per year using either a handheld petrol strimmer or a single-axle mower with a double-knife cutter bar. The study was conducted during the establishment phase of the cultivation sites and therefore Sphagnum mosses had not yet been harvested by the end of this study.
Data acquisition
Plant species numbers and cover were assessed at both study sites in September 2018 in plots of 25 × 25 cm. 247 plots (DRT: 126, PRM: 121) were distributed systematically along transects. The transects were positioned with a distance of 10 m perpendicular to the irrigation ditches (Fig. 1). In each plot, the number of all present plant species (vascular plants, Sphagnum, other bryophytes) was recorded. The cover (%) of plant groups (total, vascular plants, Sphagnum, other bryophytes) as well as the cover of each individual plant species were estimated according to the scale of Londo (1976).
The minimum distance of each plot to an irrigation ditch (m) and the peat layer thickness (cm) were measured as possible influencing variables, as both could influence the supply of the irrigation water to the growing point. The thickness of the residual peat layer was measured with a metal rod, labelled with a scale, at 18 points at DRT and 10 points at PRM. Each plot was assigned with the peat layer thickness of the nearest measuring point.
Data analysis
All statistical analyses were performed with R software (R version 4.0.3, R Development Core Team 2020). First, normality and homogeneity of variance were tested using the Shapiro-Wilk Test (Shapiro and Wilk 1965) and the Levene Test (Levene 1960), respectively.
Differences between the study sites in the cover of plant groups (total, vascular plant, Sphagnum, other bryophytes) and the number of species per plot were tested with the non-parametric Wilcoxon Rank Sum Test (Mann-Whitney U test) (Wilcoxon 1945) (significance level of p < 0.05) because the data was not normally distributed. The test was implemented using R package “ggpubr” (Kassambra 2020).
To determine which factors influence overall vascular plant cover and plant species numbers, six variables were tested for their influence. Three categorical variables resulting from the installation of the cultivation sites were included in the analysis. First, the site (location) which represents different site conditions and rewetting history (DRT: establishment directly after peat extraction, PRM: rewetted seven years prior), second, the donor species (S. papillosum, S. palustre) that had been introduced to the cultivation sites, which includes different months of introduction and different time since introduction (S. papillosum: fall 2015, 36 months; S. palustre: spring 2016, 30 months), and third the type of protective cover (straw, geotextile). In addition, three measured numerical variables representing environmental conditions at the sites were included: The Sphagnum cover (%), the thickness of the residual peat layer (cm) and the minimum distance to an irrigation ditch (m).
For graphical presentation of the relationships between the variables and vascular plant cover as well as plant species numbers, all six possible influencing variables were plotted against the recorded overall vascular plant cover and number of species per plot determined at all 247 observations at both sites. Boxplots were used for the graphical presentation of the three categorical variables. Jitter point plots were used for the three numerical variables. Jitter plots are a variant of a scatter plot where overlapping data points are better visualised by slightly shifting the points along the axes. All plots were visualised with R package “ggplot2” (Wickham 2016).
To test the influence of the six variables on vascular plant cover and the number of species per plot, boosted regression trees (BRTs) using the R packages “dismo” (Hijmans et al. 2017) and “gbm” (Greenwell et al. 2019) were used. With this method, multiple regression models (regression trees) are calculated, and an adaptive method is applied to combine several simple models to improve the prediction performance (boosting) (Elith et al. 2008). BRTs have no formal distributional assumptions. They can use non-parametric data, both categorical and numerical variables and fit complex nonlinear relationships. Approaches described in Bechtold et al. (2014) were applied to avoid overfitting (monotonic slopes, dropping of correlated variables). The training models were performed with all 274 observations and all six predictor variables, using the Gaussian distribution with tree complexity = 4, learning rate = 0.002 and bag fraction = 0.5. Only the three most influential predictors (factors) were used for the final models.
Results
Plant cover and species numbers
The total plant cover was significantly higher at PRM (mean: 84%) compared to DRT (mean: 38%) (Table 1). This results from both higher vascular plant and Sphagnum cover at PRM. Vascular plant cover had a mean of 45% at PRM and 15% at DRT (Table 1; Fig. 2a). The dominant species were Eriophorum angustifolium, Erica tetralix, Molinia caerulea and Rhynchospora alba (Table 2). They all had a high frequency in the plots but mostly a low cover (mean: 2–4%). Only two species, E. tetralix (mean: 9%) and E. angustifolium (mean: 25%) had a higher cover at PRM. Of these the latter was the dominant vascular plant species at PRM. The Sphagnum carpet had a mean cover of 60% at PRM and 22% at DRT (Table 1). The dominant Sphagnum species at both sites were the target species S. papillosum and S. palustre. Additionally, S. cuspidatum had a high frequency in the plots at PRM (91 of n = 121) but only low cover (mean: 7%) (Table 2). The cover of bryophytes besides Sphagnum was low at both sites (mean: 1% at (PRM), 5% (DRT)), but some were rare and threatened typical bog species (e.g. Kurzia pauciflora).
In all plots, a total of 24 plant species at PRM (10 vascular, 3 Sphagnum, 11 other bryophytes) and 27 plant species at DRT (13 vascular, 4 Sphagnum, 10 other bryophytes) were found. 20 of these species at each site were typical bog plant species, 14 species of which were threatened or near-threatened (Table 2).
The number of plant species per plot was significantly higher at PRM (mean: 5.1) compared to DRT (mean: 3.7) (Table 1; Fig. 3a). Also, the number of vascular plant species in total, Sphagnum and other typical bog species per plot was higher at PRM. The number of bryophyte and bog tolerant species per plot was higher at DRT. While the number of threatened species per plot did not differ significantly between the sites, the number of near-threatened species per plot was higher at PRM and the number of not threatened species per plot was higher at DRT.
Factors influencing vascular plant cover
Vascular plant cover was higher at PRM (mean: 45%) compared to DRT (mean: 15%) (Fig. 2a). Sections at PRM that were introduced with the donor species S. palustre had a higher vascular plant cover (mean: 52%) than S. papillosum (mean: 42%). At DRT sections with introduced S. papillosum (mean: 18%) had a higher vascular plant cover than sections that were introduced with S. palustre (mean: 11%) (Fig. 2b). Regarding the used protective cover, vascular plant cover was highest at PRM in sections that were covered with straw (mean: 49%) and lowest in sections that were covered with geotextile at DRT (mean: 13%) (Fig. 2c).
Low vascular plant cover was associated with low Sphagnum cover. Higher values of vascular plant cover were often found when Sphagnum cover is also high (Fig. 2d). Peat layer thickness does not show a clear correlation with vascular plant cover. However, the distribution of the measured values showed that while at DRT peat layer thickness varied between 32 and 100 cm, at PRM the residual peat layer thickness was 86–100 cm (Fig. 2e). Values of lower vascular plant cover are clustered with low distance to an irrigation ditch (Fig. 2f).
The BRT model for vascular plant cover was fitted with 1250 trees and reached a total explained deviance of 0.41 as well as a Nash-Sutcliffe efficiency of 0.28. The model indicates that the three most influential predictors (factors) for vascular plant cover were the distance to an irrigation ditch (m), the site (location) and Sphagnum cover (%). The factor with the highest relative influence on vascular plant cover was the distance to an irrigation ditch (41.4%). With a greater distance of the growing point to the nearest irrigation ditch, the vascular plant cover also increased (Fig. 2g). However, after a distance of more than 10 m, vascular plant cover slightly decreases again. The factor site had a relative influence on vascular plant cover of 32.5% (Fig. 2h). The site PRM was associated with a much higher vascular plant cover compared to DRT. The relative influence of the factor Sphagnum cover was 26.1% (Fig. 2i). Lower cover of Sphagnum (up to 40%) was associated with lower cover of vascular plants as well. Sphagnum cover above 40% positively influences vascular plant cover. However, if Sphagnum cover is above 90%, vascular plant cover shows lower values again.
Boxplots and jitter plots showing the cover of vascular plants (%) in the plots at the two cultivation sites with the dependence on six variables which were used for the BRT model. Boxplots (categorical variables, a–c) are showing median (central thick lines), 25 and 75% quartile ranges around the median (box length), outliers (circles) and mean values (rhomb). n = number of plots. Jitter plots (numerical variables, d–f) show each measured value as a point, which were slightly shifted for better visualisation of overlapping data points. a cultivation site (DRT, PRM), b donor species (this variables includes different months of introduction and different time since introduction, S. papillosum: fall 2015, 36 months; S. palustre: spring 2016, 30 months), c type of protective cover (straw, geotextile), d Sphagnum cover (%), e thickness of the residual peat layer (cm), f the minimum distance to an irrigation ditch (m). Boosted regression tree partial dependence plot showing the effect of the three most influential predictors (factors) on the cover of vascular plants (%) at the two cultivation sites DRT (n = 126) and PRM (n = 121). g distance to irrigation ditch (m), h the cultivation site and i Sphagnum cover (%). The fitted function is the difference between the actual y-axis value and the mean response value. The important predictor range is where the fitted function is above zero. The graphs show the effect of a particular variable on the response: positive fitted function values suggest that vascular plant cover responds favourably and low values suggest the opposite. The relative influence of each factor on vascular plant cover is shown in parentheses below each graph.
Factors influencing plant species numbers
The number of plant species per plot was higher at PRM (mean: 5.1) than at DRT (mean: 3.7) (Fig. 3a). At DRT, the number of species per plot was higher in sections that were introduced with donor species S. papillosum (mean: 4.0) compared to S. palustre (mean: 3.2) (Fig. 3b). At PRM, it was similar between the sections with introduction of S. papillosum (mean: 5.1) and S. palustre (mean: 4.9). The highest number of species per plot was found at PRM in sections covered with geotextile (mean: 5.3), while the lowest numbers were found at DRT, when the same protective cover was used (mean: 3.1) (Fig. 3c).
High numbers of species per plot were found more often when Sphagnum cover was high (Fig. 3d). Above a Sphagnum cover of 30% the number of species was rarely lower than 2.5 species per plot. A clear correlation between peat layer thickness and number of species per plot is not discernible (Fig. 3e). Where the distance to an irrigation ditch was greater than 5 m the number of species was never lower than 2.5 per plot (Fig. 3f).
The BRT model for the number of plant species per plot was fitted with 1300 trees and reached a total explained deviance of 0.40 as well as a Nash-Sutcliffe efficiency of 0.27. The model identified the three most influential predictors (factors) for the number of species per plot as Sphagnum cover (%), the distance to an irrigation ditch (m) and the donor species. The highest relative influence was found with Sphagnum cover (52.6%), which positively correlated with the number of species per plot (Fig. 3g). The factor distance to irrigation ditch had a relative influence of 34.8% and the number of species per plot responds positively to a distance greater than 15 m (Fig. 3h). The donor species S. papillosum was associated with a higher number of species per plot than S. palustre and had a relative influence of 12.6% (Fig. 3i).
Boxplots and jitter plots showing the number of plant species per plot at the two cultivation sites with the dependence on six variables which were used for the BRT model. Boxplots (categorical variables, a–c) are showing median (central thick lines), 25 and 75% quartile ranges around the median (box length), outliers (circles) and mean values (rhomb). n = number of plots. Jitter plots (categorical variables, d–f) show each measured value as a point, which were slightly shifted for better visualisation of overlapping data points. a cultivation site (DRT, PRM), b donor species (this variable includes different months of introduction and different time since introduction, S. papillosum: fall 2015, 36 months; S. palustre: spring 2016, 30 months), c type of protective cover (straw, geotextile), d Sphagnum cover (%), e thickness of the residual peat layer (cm), f the minimum distance to an irrigation ditch (m). Boosted regression tree partial dependence plot showing the effect of the three most influential predictors (factors) on the number of species per plot at the two cultivation sites DRT (n = 126) and PRM (n = 121). g Sphagnum cover (%), h the minimum distance to an irrigation ditch and i the donor species. The fitted function is the difference between the actual y-axis value and the mean response value. The important predictor range is where the fitted function is above zero. The graphs show the effect of a particular variable on the response: positive fitted function values suggest that the number of species per plot responds favourably and low values suggest the opposite. The relative influence of each factor on the number of species per plot is shown in parentheses below each graph.
Discussion
Factors influencing vascular plant cover
Vascular plant cover was significantly higher at the cultivation site that was rewetted seven years prior to establishment (PRM, mean: 45%) than at the site that was rewetted concurrent with its establishment (DRT, mean: 15%). Accordingly, the site (location) was identified with the BRT model as a factor influencing vascular plant cover. While no vascular plant species had a higher mean cover than 4% at DRT, Erica tetralix (9%) and especially Eriophorum angustifolium (25%) had a comparatively high mean cover at PRM. E. angustifolium colonises rewetted peatlands quickly, because its seeds spread well with the wind (Salonen 1994; Campbell et al. 2003; Lavoie et al. 2005). The colonisation potential of this species was high at PRM, because it had already established abundantly in the surroundings of the site during the previous seven years of rewetting (data not shown). At DRT, no vascular plant species were abundant in the surrounding areas that could have migrated to the cultivation site as these were mostly bare and active peat extraction sites. These results indicate the importance of considering the colonisation potential of other plant species from the site’s surroundings when selecting the location for a cultivation site.
The distance of the growing point to the nearest irrigation ditch had the greatest effect on vascular plant cover which increases with growing distance. This suggests irrigation water not sufficiently reaching the centre of the sites, resulting in drier conditions promoting vascular plant establishment. The insufficient water distribution can be explained by the low hydraulic conductivity of the highly decomposed black peat (Liu and Lennartz 2019). Additionally, the water table dropped lower in summer at PRM (down to 80 cm below peat surface) than at DRT (down to 55 cm below) because irrigation water ran out during dry summers. This may also have promoted higher vascular plant cover at PRM. Restoration sites with unfavourable hydrological conditions (insufficient rewetting, water table fluctuations) are known to be colonised by vascular plants rather than Sphagnum (Lavoie and Rochefort 1996; Girard et al. 2002; Lavoie et al. 2003; Vasander et al. 2003; Lanta et al. 2004; Poulin et al. 2005). Therefore, sufficient water supply (stable, near-surface water table throughout the year) will not only facilitate optimal Sphagnum growth. It may also reduce vascular plant cover, when a closed Sphagnum carpet with a cover greater than 90% is achieved and thus reduces competition and the requirement for mowing. For this purpose, reserves should be provided for years with low precipitation and dry summers. Depending on the hydraulic conductivity of the peat, the spacing of irrigation structures should be designed for optimal distribution of irrigation water.
Near irrigation structures where water availability is good, Sphagnum mosses also show better growth rates (Gaudig et al. 2017; Grobe et al. 2021). The BRT model shows that low Sphagnum cover is associated with low vascular plant cover. Especially in concert with insufficient water supply, temperature fluctuations, high evaporation and wind erosion can render the conditions on bare peat difficult for plant establishment (Salonen 1994; Campeau and Rochefort 1996; Price 1996; Campbell et al. 2002). While Sphagnum cover above 40% has a positive effect on vascular plant cover initially, Sphagnum cover above 90% influences vascular plant cover negatively. In areas where Sphagnum grows well, vascular plant productivity is often reduced. Under optimal conditions Sphagnum can even overgrow vascular plants (Backéus 1985; Malmer et al. 1994; Rydin et al. 2006). Thus, where optimal Sphagnum growth is achieved, it is also likely that vascular plant cover will be reduced or kept low.
Factors influencing plant species numbers
The total number of plant species and the number of typical bog species per plot was significantly higher at PRM (mean: 5.1 & 5.0) compared to DRT (mean: 3.7 & 3.4). The higher numbers at PRM could be explained by the migration of species from the rewetted surroundings that established at the site in addition to the species transferred with the donor material. Nevertheless, in the BRT model, the factor site (location) was not influencing the number of species per plot. The number of threatened species per plot (e.g. Vaccinium oxycoccos, Drosera rotundifolia) did not differ significantly between sites. Rhynchospora alba and Drosera intermedia were even more frequent in the plots at DRT than at PRM. This can be explained by the low Sphagnum cover at DRT and the associated high bare peat cover (Grobe et al. 2021), as both species like to colonise bare peat (Rybníček 1970; Thum 1986). The number of bog tolerant species per plot was low at both sites (PRM, mean: 0.1 & DRT, mean: 0.3) which has also been found true for establishing vegetation at abandoned cut-over peatlands (Girard et al. 2002; Poulin et al. 2005). This indicates that the conditions were instead better suitable for typical bog species. Hence, non-typical species are unlikely to establish with high frequency at the sites or may not survive there for long. The frequent species that were present in more than ten plots were found at both sites. Exceptions to this are Calluna vulgaris, which was only frequent at PRM, as well as Juncus bulbosus and J. effuses, which were only relatively frequent at DRT. The presence of Juncus species that are tolerant to higher nutrient concentrations may be attributed to the additional use of groundwater at DRT with higher nutrient levels. This was also observed at another cultivation site by Temmink et al. (2017). Where dominant, Juncus species can reduce cover and numbers of other species (Ervin and Wetzel 2002). They could thus become competition for Sphagnum in more nutrient-rich conditions. Therefore, nutrient-poor water (rainwater) should be used to prevent the dominance of competitive, nutrient-tolerant plant species.
The factor with the greatest positive influence on the number of plant species per plot determined by the BRT model was Sphagnum cover. This suggests that where the conditions at the cultivation sites were suitable for Sphagnum, they were also suitable for other, primarily typical bog plant species. However, when Sphagnum cover exceeds 90%, the number of species per plot decreased. Sphagnum mosses can suppress other species by keeping their habitat wet and acidic (Rydin et al. 2006). At cultivation sites, the aim is to establish a closed Sphagnum carpet (Pouliot et al. 2015; Gaudig et al. 2018). If this aim is achieved, a lower number of species can be expected compared to sites where structural diversity results from uneven establishment of Sphagnum (as for the sites of this study).
A distance of more than 15 m of the growing point to the nearest irrigation ditch had a positive influence on the number of plant species per plot, as it did on vascular plant cover. Again, it can be assumed that with greater distance and thus poorer water supply, additional species establish that are more tolerant to drier conditions. Thus, when homogeneous sites with good water supply are established, the number of species potentially present at the site will be reduced.
The applied donor material was also a factor influencing the number of plant species per plot. In the areas where donor material with S. papillosum was used, a higher number of species per plot was found than in the areas where S. palustre was introduced. A correlation between species composition at the recipient site and its donor site was also observed for restoration sites that were introduced with Sphagnum biomass (Karofeld et al. 2016; Hugron et al. 2020). However, for this study, it should be considered that the donor material with S. papillosum was introduced about half a year earlier (October 2015) than S. palustre (March 2016) and because of this, species had more time to establish. Nevertheless, the differing half-year was not part of the growing season. Following these considerations, the number and composition of species at a cultivation site can be influenced by the selection of the donor site.
Conclusion
Summarising, the main factors influencing vascular plant cover at the two Sphagnum cultivation sites were the distance to an irrigation ditch (m), the site (location, which represents the different site conditions and rewetting history), and Sphagnum cover (%). The number of plant species per plot was influenced mainly by Sphagnum cover (%), the distance to an irrigation ditch (m) and the donor species. Whether the reduction or mowing of vascular plants at cultivation sites is necessary depends on the end use of the cultivated Sphagnum biomass, the site conditions as well as the cover, the plant species, and the amount of litter it produces (Guêné-Nanchen et al. 2017; Gaudig et al. 2017, 2018). The cover might also decrease with succession as observed by Guêné-Nanchen et al. (2017). It is therefore a case-by-case decision.
The results of this study and their interpretation apply to the study sites but may only be adopted to other sites to a limited extend, as true replications could not be realised due to the pre-existing site design. However, from the observations at the two cultivation sites of this study, the following conclusions and recommendations for the management of vascular plant cover and plant species numbers at Sphagnum cultivation sites can be derived:
-
The colonisation potential of other plant species from the site’s surroundings needs to be considered when selecting the location for a cultivation site.
-
Sufficient water supply (stable, near-surface water table throughout the year) will not only facilitate optimal Sphagnum growth but may also reduce vascular plant cover and the number of plant species potentially present at a site (e.g. species tolerant to drier conditions) and thus reduce competition and the requirement for mowing. Water reserves should be provided for years with low precipitation and dry summers. The spacing of irrigation structures should be designed for optimal distribution of irrigation water depending on the local hydraulic conductivity of the peat.
-
Nutrient-poor water (rainwater) should be used to prevent the dominance of competitive, nutrient-tolerant plant species (e.g. Juncus species).
-
When the conditions are suitable for Sphagnum, they are also suitable for other typical bog plant species and it is unlikely for non-typical species to establish permanently at the sites.
-
When optimal Sphagnum growth and a closed Sphagnum carpet are achieved, vascular plant cover will be reduced or kept low and a lower number of plant species can be expected compared to sites where structural diversity results from uneven establishment of Sphagnum.
-
The number and composition of plant species at a cultivation site can be influenced by the selection of the donor site.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Backéus I (1985) Aboveground production and growth dynamics of vascular bog plants in central Sweden. Acta phytogeographica Suecica, vol 74. Svenska växtgeografiska sällskapet; Almqvist & Wiksell International [distributor], Uppsala, Stockholm, Uppsala University
Beaulne J, Garneau M, Magnan G, Boucher É (2021) Peat deposits store more carbon than trees in forested peatlands of the boreal biome. Sci Rep 11:1–11. https://doi.org/10.1038/s41598-021-82004-x
Bechtold M, Tiemeyer B, Laggner A, Leppelt T, Frahm E, Belting S (2014) Large-scale regionalization of water table depth in peatlands optimized for greenhouse gas emission upscaling. Hydrol Earth Syst Sci 18:3319–3339. https://doi.org/10.5194/hess-18-3319-2014
Beyer C, Höper H (2015) Greenhouse gas exchange of rewetted bog peat extraction sites and a Sphagnum cultivation site in northwest Germany. Biogeosciences 12:2101–2117. https://doi.org/10.5194/bg-12-2101-2015
Blankenburg J (2004) Praktische Hinweise zur optimalen Wiedervernässung von Torfabbauflächen. Geofakten 14:1–11
Campbell DR, Lavoie C, Rochefort L (2002) Wind erosion and surface stability in abandoned milled peatlands. Can J Soil Sci 82:85–95. https://doi.org/10.4141/S00-089
Campbell DR, Rochefort L, Lavoie C (2003) Determining the immigration potential of plants colonizing disturbed environments: the case of milled peatlands in Quebec. J Appl Ecol 40:78–91. https://doi.org/10.1046/j.1365-2664.2003.00782.x
Campeau S, Rochefort L (1996) Sphagnum regeneration on bare peat surfaces: field and greenhouse experiments. J Appl Ecol 33:599–608. https://doi.org/10.2307/2404988
DWD (2022) German Weather Service: mean values for the period 1981–2010 (German). https://www.dwd.de/DE/leistungen/klimadatendeutschland/vielj_mittelwerte.html. Accessed 14 March 2022
Elith J, Leathwick JR, Hastie T (2008) A working guide to boosted regression trees. J Anim Ecol 77:802–813. https://doi.org/10.1111/j.1365-2656.2008.01390.x
Ellenberg H, Leuschner C (2010) Vegetation of central europe with the alps (German), 6th edn. Verlag Eugen Ulmer, Stuttgart
Emmel M (2008) Growing ornamental plants in Sphagnum biomass. ISHS Acta Hortic Proc Int Symp Grow Media 779:173–178. https://doi.org/10.17660/ACTAHORTIC.2008.779.20
Ervin GN, Wetzel RG (2002) Influence of a dominant macrophyte, Juncus effusus, on wetland plant species richness, diversity, and community composition. Oecologia 130:626–636. https://doi.org/10.1007/s00442-001-0844-x
Frahm J-P, Frey W (2004) Mossflora (German), 4th edn. UTB Ulmer, Stuttgart
Garve E (2004) Red list and flora list of vascular plants in Lower Saxony and Bremen (German). Informationsdienst Naturschutz Niedersachsen 24:1–76
Gaudig G, Fengler F, Krebs M, Prager A, Schulz J, Wichmann S, Joosten H (2014) Sphagnum farming in Germany—a review of progress. Mires Peat 13:1–11
Gaudig G, Krebs M, Joosten H (2017) Sphagnum farming on cut-over bog in NW Germany: long-term studies on Sphagnum growth. Mires Peat 20:1–19. https://doi.org/10.19189/MaP.2016.OMB.238
Gaudig G, Krebs M, Prager A, Wichmann S, Barney M, Caporn SJ, Emmel M, Fritz C, Graf M, Grobe A, Gutierrez Pacheco S, Hogue-Hugron S, Holzträger S, Irrgang S, Kämäräinen A, Karofeld E, Koch G, Köbbing JF, Kumar S, Matchutadze I, Oberpaur C, Oestmann J, Raabe P, Rammes D, Rochefort L, Schmilewski G, Sendžikaitė J, Smolders A, St-Hilaire B, van de Riet B, Wright B, Wright N, Zoch L, Joosten H (2018) Sphagnum farming from species selection to the production of growing media: a review. Mires Peat 20:1–30. https://doi.org/10.19189/MaP.2018.OMB.340
Gaudig G, Krebs M, Joosten H (2020) Sphagnum growth under N saturation: interactive effects of water level and P or K fertilization. Plant Biol 22:394–403. https://doi.org/10.1111/plb.13092
Girard M, Lavoie C, Thériault M (2002) The regeneration of a highly disturbed ecosystem: a mined Peatland in Southern Québec. Ecosyt 5:274–288. https://doi.org/10.1007/s10021-001-0071-7
González E, Rochefort L (2014) Drivers of success in 53 cutover bogs restored by a moss layer transfer technique. Ecol Eng 68:279–290. https://doi.org/10.1016/j.ecoleng.2014.03.051
Gorham E (1991) Northern Peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl: Public Ecol Soc Am 1:182–195. https://doi.org/10.2307/1941811
Greenwell B, Boehmke B, Cunningham J, Developers GBM (2019) R package “gbm”: generalized boosted regression models. Version 2.1.5. https://CRAN.R-project.org/package=gbm
Grobe A, Tiemeyer B, Graf M (2021) Recommendations for successful establishment of Sphagnum farming on shallow highly decomposed peat. Mires Peat 27:1–18. https://doi.org/10.19189/MaP.2020.APG.StA.2022
Guêné-Nanchen M, Pouliot R, Hugron S, Rochefort L (2017) Effect of repeated mowing to reduce graminoid plant cover on the moss carpet at a Sphagnum farm in North America. Mires Peat 20:1–12. https://doi.org/10.19189/MaP.2016.OMB.250
Günther A, Jurasinski G, Albrecht K, Gaudig G, Krebs M, Glatzel S (2017) Greenhouse gas balance of an establishing Sphagnum culture on a former bog grassland in Germany. Mires Peat 20:1–16. https://doi.org/10.19189/MaP.2015.OMB.210
Harenda KM, Lamentowicz M, Samson M, Chojnicki BH (2018) The role of peatlands and their carbon storage function in the context of climate change. GeoPlanet Earth Planetary Sci. https://doi.org/10.1007/978-3-319-71788-3_12
Hayward PM, Clymo RS (1983) The growth of Sphagnum: experiments on, and simulation of, some effects of light flux and water-table depth. J Ecol 71:845–863. https://doi.org/10.2307/2259597
Hijmans RJ, Phillips S, Leathwick J, Elith J (2017) R package “dismo”: species distribution modeling. Version 1.1-4. https://CRAN.R-project.org/package=dismo
Hugron S, Rochefort L (2018) Sphagnum mosses cultivated in outdoor nurseries yield efficient plant material for peatland restoration. Mires Peat 20:1–6. https://doi.org/10.19189/MaP.2018.OMB.358
Hugron S, Guêné-Nanchen M, Roux N, LeBlanc M-C, Rochefort L (2020) Plant reintroduction in restored peatlands: 80% successfully transferred—does the remaining 20% matter? Glob Ecol Conserv 22:e01000. https://doi.org/10.1016/j.gecco.2020.e01000
Jäger E et al (eds) (2011) Excursion Flora of Germany (German), 20th edn. Spektrum Akademischer Verlag, Heidelberg
Jobin P, Caron J, Rochefort L (2014) Developing new potting mixes with Sphagnum fibers. Can J Soil Sci 94:585–593. https://doi.org/10.4141/cjss2013-103
Joosten H (2012) Zustand und Perspektiven der Moore weltweit. Natur und Landschaft 87:50–55
Joosten H, Tanneberger F, Moen A (2017) Mires and peatlands of Europe: status, distribution and conservation. Schweizerbart Science Publishers, Stuttgart
Karofeld E, Müür M, Vellak K (2016) Factors affecting re-vegetation dynamics of experimentally restored extracted peatland in Estonia. Environ Sci Pollut Res Int 23:13706–13717. https://doi.org/10.1007/s11356-015-5396-4
Karofeld E, Jarašius L, Priede A, Sendžikaitė J (2017) On the after-use and restoration of abandoned extracted peatlands in the baltic countries. Restor Ecol 25:293–300. https://doi.org/10.1111/rec.12436
Kassambara A, Mundt F (2020) R package “factoextra”: extract and visualize the results of multivariate data analyses. Version 1.0.7. https://CRAN.R-project.org/package=factoextra
Konvalinková P, Prach K (2014) Environmental factors determining spontaneous recovery of industrially mined peat bogs: a multi-site analysis. Ecol Eng 69:38–45. https://doi.org/10.1016/j.ecoleng.2014.03.090
Koperski M (2011) Red List and complete species list of mosses in Lower Saxony and Bremen (German). Informationsdienst Naturschutz Niedersachsen 31:131–205
Krebs M, Gaudig G, Matchutadze I, Joosten H (2018) Sphagnum regrowth after cutting. Mires Peat 12:1–20. https://doi.org/10.19189/MaP.2017.OMB.298
Kumar S (2017) Sphagnum moss as a growing media constituent: some effects of harvesting, processing and storage. Mires Peat 20:1–11. https://doi.org/10.19189/MaP.2016.OMB.232
Laine A, Byrne KA, Kiely G, Tuittila E-S (2007) Patterns in vegetation and CO2 dynamics along a water level gradient in a lowland blanket bog. Ecosyst 10:890–905. https://doi.org/10.1007/s10021-007-9067-2
Lanta V, Doležal J, Šamata J (2004) Vegetation patterns in a cut-away peatland in relation to abiotic and biotic factors: a case study from the Šumava Mts., Czech Republic. Suo 55:33–43
Lavoie C, Rochefort L (1996) The natural revegetation of a harvested peatland in southern Québec: a spatial and dendroecological analysis. Écoscience 3:101–111
Lavoie C, Grosvernier P, Girard M, Marcoux K (2003) Spontaneous revegetation of mined peatlands: an useful restoration tool? Wetlands Ecol Manag 11:97–107. https://doi.org/10.1023/A:1022069808489
Lavoie C, Saint-Louis A, Lachance D (2005) Vegetation dynamics on an abandoned vacuum-mined peatland: 5 years of monitoring. Wetlands Ecol Manag 13:621–633. https://doi.org/10.1007/s11273-005-0126-1
Levene H (1960) Robust tests for equality of variances. In: Olkin I, Hotelling H (eds) Contributions to probability and statistics. Stanford University Press, Palo Alto, pp 278–292
Liu H, Lennartz B (2019) Hydraulic properties of peat soils along a bulk density gradient-a meta study. Hydrol Process 33:101–114. https://doi.org/10.1002/hyp.13314
Loisel J, Gallego-Sala AV, Amesbury MJ, Magnan G, Anshari G, Beilman DW, Benavides JC, Blewett J, Camill P, Charman DJ, Chawchai S, Hedgpeth A, Kleinen T, Korhola A, Large D, Mansilla CA, Müller J, van Bellen S, West JB, Yu Z, Bubier JL, Garneau M, Moore T, Sannel ABK, Page S, Väliranta M, Bechtold M, Brovkin V, Cole LES, Chanton JP, Christensen TR, Davies MA, de Vleeschouwer F, Finkelstein SA, Frolking S, Gałka M, Gandois L, Girkin N, Harris LI, Heinemeyer A, Hoyt AM, Jones MC, Joos F, Juutinen S, Kaiser K, Lacourse T, Lamentowicz M, Larmola T, Leifeld J, Lohila A, Milner AM, Minkkinen K, Moss P, Naafs BDA, Nichols J, O’Donnell J, Payne R, Philben M, Piilo S, Quillet A, Ratnayake AS, Roland TP, Sjögersten S, Sonnentag O, Swindles GT, Swinnen W, Talbot J, Treat C, Valach AC, Wu J (2021) Expert assessment of future vulnerability of the global peatland carbon sink. Nat Clim Chang 11:70–77. https://doi.org/10.1038/s41558-020-00944-0
Londo G (1976) The decimal scale for releves of permanent quadrats. Vegetation 33:61–64. https://doi.org/10.1007/BF00055300
Malmer N, Svensson BM, Wallén B (1994) Interactions between Sphagnum mosses and field layer vascular plants in the development of peat-forming systems. Folia Geobot Phytotax 29:483–496. https://doi.org/10.1007/BF02883146
Money RP (1994) Restoration of lowland raised bogs damaged by peat extraction—with particular emphasis on Sphagnum regeneration. Thesis for the degree of Doctor of Philosophy, University of Sheffield
Müller R, Glatzel S (2021) Sphagnum farming substrate is a competitive alternative to traditional horticultural substrates for achieving desired hydro-physical properties. Mires Peat 27:1–12. https://doi.org/10.19189/MaP.2021.OMB.StA.2157
Oberpaur C, Puebla V, Vaccarezza F, Arévalo ME (2010) Preliminary substrate mixtures including peat moss (Sphagnum magellanicum) for vegetable crop nurseries. Cienc Inv Agr 37. https://doi.org/10.4067/S0718-16202010000100012
Oestmann J, Tiemeyer B, Düvel D, Grobe A, Dettmann U (2021) Greenhouse gas balance of sphagnum farming on highly decomposed peat at former peat extraction sites. Ecosystem 25:350–371. https://doi.org/10.1007/s10021-021-00659-z
Poulin M, Rochefort L, Quinty F, Lavoie C (2005) Spontaneous revegetation of mined peatlands in eastern Canada. Can J Bot 83:539–557. https://doi.org/10.1139/b05-025
Pouliot R, Rochefort L, Karofeld E, Mercier C (2011) Initiation of Sphagnum moss hummocks in bogs and the presence of vascular plants: is there a link? Acta Oecol 37:346–354. https://doi.org/10.1016/j.actao.2011.04.001
Pouliot R, Hugron S, Rochefort L (2015) Sphagnum farming: a long-term study on producing peat moss biomass sustainably. Ecol Eng 74:135–147. https://doi.org/10.1016/j.ecoleng.2014.10.007
Price JS (1996) Hydrology and microclimate of a partly restored cutover bog. Québec Hydrol 10:1263–1272
Quinty F, Rochefort L (2003) Peatland restoration guide, 2nd edn. Canadian Sphagnum Peat Moss Association and New Brunswick Department of Natural Resources and Energy, Québec
R Development Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Robroek BJ, van Ruijven J, Schouten MG, Breeuwer A, Crushell PH, Berendse F, Limpens J (2009) Sphagnum re-introduction in degraded peatlands: the effects of aggregation, species identity and water table. Basic Appl Ecol 10:697–706. https://doi.org/10.1016/j.baae.2009.04.005
Rybníček K (1970) Rhynchospora alba (L.) Vahl, its distribution, communities and habitat conditions in Czechoslovakia, Part 2. Folia Geobotanica et Phytotaxonomica 5:221–263
Rydin H, Jeglum JK (2006) The biology of peatlands. Oxford University Press, New York
Rydin H, Gunnarsson U, Sundberg S (2006) The role of Sphagnum in peatland development and persistence. In: Wieder RK, Vitt DH (eds) Boreal peatland ecosystems: ecological studies. Springer, Berlin, pp 47–65
Salonen V (1994) Revegetation of harvested peat surfaces in relation to substrate quality. J Veg Sci 5:403–408. https://doi.org/10.2307/3235863
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (for complete samples). Biometrika 52:591–611. https://doi.org/10.1093/biomet/52.3-4.591
Smolders A, Tomassen H, van Mullekom M, Lamers L, Roelofs J (2003) Mechanisms involved in the re-establishment of Sphagnum-dominated vegetation in rewetted bog remnants. Wetlands Ecol Manag 11:403–418. https://doi.org/10.1023/B:WETL.0000007195.25180.94
Temmink RJ, Fritz C, van Dijk G, Hensgens G, Lamers LP, Krebs M, Gaudig G, Joosten H (2017) Sphagnum farming in a eutrophic world: the importance of optimal nutrient stoichiometry. Ecol Eng 98:196–205. https://doi.org/10.1016/j.ecoleng.2016.10.069
Thum M (1986) Segregation of habitat and prey in two sympatric carnivorous plant species, Drosera rotundifolia and Drosera intermedia. Oecologia 70:601–605. https://doi.org/10.1007/BF00379912
Tiemeyer B, Freibauer A, Borraz EA, Augustin J, Bechtold M, Beetz S, Beyer C, Ebli M, Eickenscheidt T, Fiedler S, Förster C, Gensior A, Giebels M, Glatzel S, Heinichen J, Hoffmann M, Höper H, Jurasinski G, Laggner A, Leiber-Sauheitl K, Peichl-Brak M, Drösler M (2020) A new methodology for organic soils in national greenhouse gas inventories: data synthesis, derivation and application. Ecol Indic 109:105838. https://doi.org/10.1016/j.ecolind.2019.105838
Triisberg T, Karofeld E, Paal J (2013) Factors affecting the re-vegetation of abandoned extracted peatlands in Estonia: a synthesis from field and greenhouse studies. Est J Ecol 62:1–20. https://doi.org/10.3176/eco.2013.3.02
Tuittila E-S, Vasander H, Laine J (2000) Impact of rewetting on the vegetation of a cut-away peatland. Appl Veg Sci 3:205–212. https://doi.org/10.2307/1478999
Vasander H, Tuittila E-S, Lode E, Lundin L, Ilomets M, Sallantaus T, Heikkilä R, Pitkänen M-L, Laine J (2003) Status and restoration of peatlands in northern Europe. Wetlands Ecol Manag 11:51–63. https://doi.org/10.1023/A:1022061622602
Von Post L (1924) The genetic system of the organogenic formations of Sweden (German). In: Comité International de Pédologie, IVème commission (commission pour la nomenclature et la classification des sols, commission pour l’Europe, président: B. Frosterus) (ed) Mémoires sur la Nomenclature et la Classification des Sols, Helsingfors/Helsinki, pp 287–304
Waddington JM, Price JS (2000) Effect of peatland drainage, harvesting and restoration on atmospheric water and carbon exchange. Phys Geogr 21:433–451. https://doi.org/10.1080/02723646.2000.10642719
Wichtmann W, Schröder C, Joosten H (2016). Paludikultur—Bewirtschaftung nasser Moore: Klimaschutz—Biodiversität -regionale Wertschöpfung. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart
Wickham H (2016) R package “ggplot2”: elegant graphics for data analysis. Springer-Verlag, New York
Wilcoxon F (1945) Individual comparisons by ranking methods. Biometrics Bull 1:80. https://doi.org/10.2307/3001968
Acknowledgements
The establishment of the cultivation sites and the accompanied research were funded by the Lower Saxony Ministry for Nutrition, Agriculture and Consumer Protection (ML, AZ 105.1-3234/1-13-3) and the German Federal Environmental Foundation (DBU, AZ 33305/01-33/0), whose support is gratefully acknowledged. The permissions were granted by the Weser-Ems office for regional state development (state mire administration) and the County Emsland facilitated the project. We thank our project partners Klasmann-Deilmann GmbH for their productive cooperation. Jan Oestmann provided data regarding water quality. Dr. Michel Bechtold’s adaptation of the BRT model and support from Dr. Bärbel Tiemeyer greatly improved the data analysis. Maike Senne has helped considerably with the assessments in the field. Their contributions are kindly appreciated.
Funding
Open Access funding enabled and organized by Projekt DEAL. The establishment of the cultivation sites and the accompanied research were funded by the Lower Saxony Ministry for Nutrition, Agriculture and Consumer Protection (ML, AZ 105.1-3234/1-13-3) and the German Federal Environmental Foundation (DBU, AZ 33305/01-33/0).
Author information
Authors and Affiliations
Contributions
AG collected and analysed the data with the support of MR. AG wrote the first draft and both authors contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grobe, A., Rode, M. Factors influencing the establishment of vascular plants at Sphagnum cultivation sites. Wetlands Ecol Manage 31, 449–465 (2023). https://doi.org/10.1007/s11273-023-09927-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-023-09927-2