Abstract
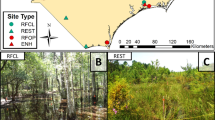
Macroinvertebrate community structure and assemblages associated with the planted, native submerged aquatic vegetation (SAV) species Heteranthera dubia (Jacq.) MacMillan and Potamogeton nodosus Poiret were examined in a series of constructed urban floodway wetlands, the Dallas Floodway Extension Lower Chain of Wetlands, Dallas, TX, USA. Macroinvertebrate community metrics, including abundance, richness, diversity, and evenness associated with SAV and three different wetlands of varying construction completion dates, water sources (direct or wetland-channeled wastewater effluent), and ecosystem management stage (established/reference or developing) were compared and analyzed. Assemblages at sampling sites were also classified and related to vegetation and wetland physicochemical parameters. Plant species affected only macroinvertebrate abundance, with the less-dissected P. nodosus supporting higher counts than H. dubia. Wetland age and water-effluent type had the most substantial effect on macroinvertebrate communities. The older, longer-managed wetland and wetland-channeled effluent habitat consistently demonstrated higher quality metrics and biodiversity than newly constructed, direct effluent wetland habitat. Increased vegetation cover and wetland age, coupled with moderate water temperature, pH, and DO levels were characteristics of more rich and diverse macroinvertebrate communities, including pollutant-sensitive taxa, such as Ephemeroptera and Trichoptera.






Similar content being viewed by others
References
Armstrong N, Planas D, Prepas E (2003) Potential for estimating macrophyte surface area from biomass. Aquat Bot 75:173–179
Balci P, Kennedy JH (2003a) Comparison of chironomids and other macroinvertebrates associated with Myriophyllum spicatum and Heteranthera dubia. J Freshw Ecol 18:187–197
Balci P, Kennedy JH (2003b) Measures of plant surface-areas for Eurasian watermilfoil and water stargrass. J Aquat Plant Manage 41:119–122
Beckett DC, Aartila TP (1991) Invertebrate abundance on Potamogeton nodosus: effects of plant surface area and condition. Can J Zool 70:300–306
Beckett DC, Aartila TP, Miller A (1992) Contrasts in density of benthic invertebrates between macrophyte beds and open littoral patches in Eau Galle Lake, Wisconsin. Am Midl Nat 127:77–90
Benke AC (1976) Dragonfly production and prey turnover. Ecology 57:915–927
Benke AC (1979) A modification of the Hynes method to estimating secondary production with particular significance for multivoltine populations. Limnol Oceanogr 24:168–171
Berg CO (1949) Limnological relations of insects to plants of the genus Potamogeton. T Am Microsc Soc 68–4:279–291
Braskerud BC, Tonderski KS, Wedding B, Bakke R, Blankerberg B, Ulen B, Koskiaho J (2005) Can constructed wetlands reduce the diffuse phosphorus loads to eutrophic water in cold temperate regions? J Environ Qual 34:2145–2155
Brown C (1988) Relationships of phytomacrofauna to surface area in naturally occurring macrophyte stands. J N Am Benthol Soc 7:129–139
Carpenter SR, Lodge DM (1986) Effects of submersed macrophytes on ecosystem processes. Aquat Bot 26:341–370
Cheruvelil K, Soranno P, Madsen J, Roberson M (2002) Plant architecture and epiphytic macroinvertebrate communities: the role of an exotic dissected macrophyte. J N Am Benthol Soc 21:261–277
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–218
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. Primer-E, Plymouth
Clarke KR, Gorley RN (2006) Primer v6: user manual/tutorial. Primer-E, Plymouth
Collier K, Champion P, Croker G (1999) Patch- and reach-scale dynamics of a macrophyte-invertebrate system in a New Zealand Lowland Stream. Hydrobiologia 392:88–97
Dick GO, Dodd LL, Schad AN, Smith DH, Owens CS (2015) Dallas floodway extension lower chain of wetlands and grasslands ecological management and monitoring. Status report to the USACE SWF. https://www.swf.usace.army.mil/Portals/47/docs/PAO/DFE/PDF/DFE_March_2015_Status_Report.pdf. Accessed Nov 2019
Downing JA, Cyr H (1985) Quantitative estimation of epiphytic invertebrate populations. Can J Fish Aquat Sci 42:1570–1579
Engel S (1988) The role and interactions of submersed macrophytes in a shallow Wisconsin Lake. J Freshwater Ecol 4:329–341
Feldmann T, Noges P (2009) Seasonal and vertical changes in the surface area/biomass ratio of Potamogeton lucens L. in a clear and turbid shallow lake. J Aquat Plant Manage 47:116–121
Gerking SH (1962) Production and food utilization in a population of bluegill sunfish. Ecol Monogr 32:31–78
Harms NE, Grodowitz M, Kennedy JH (2011) Insect Herbivores of Water Stargrass (Heteranthera dubia) in the U.S. J Freshw Ecol 16:185–194
Heino J (2000) Lentic macroinvertebrate assemblage structure along gradients in spatial heterogeneity, habitat size and water chemistry. Hydrobiologia 418:229–242
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432
Honnell DR, Madsen JD, Smart RM (1993) Effects of selected exotic and native aquatic plant communities on water temperature and dissolved oxygen. Info Exchange Bull A-93-2. U.S Army Engineer WES, Vicksburg
Jackson D (1993) Multivariate analysis of benthic invertebrate communities: the implication of choosing particular data standardizations, measures of association, and ordination methods. Hydrobiologia 268:9–26
James WF, Barko JW (1990) Macrophyte influences on the zonation of sediment accretion and composition in a north-temperate reservoir. Arch Hydrobiol 120:129–142
James WF, Barko JW (1995) Effects of submersed macrophytes on sediment resuspension in March Lake, Minnesota. In: Proceedings, 29th annual meeting, APCRP, 168–175. Miscellaneous Paper A-95-3, U.S. Army Engineer WES, Vicksburg, MS.
Keast A (1984) The introduced aquatic macrophyte, Myriophyllum spicatum, as habitatfor fish and their invertebrate prey. Can J Zool 62:1289–1303
Kenkel NC, Orloci L (1986) Applying metric and nonmetric multidimensional scaling to ecological studies: some new results. Ecology 67:919–928
Kentula ME (1999) Wetland restoration and creation National water summary on wetland resources. USGS Water-Supply Paper 2425:87–92
Krull JN (1970) Aquatic plant-macroinvertebrate associations and waterfowl. J Wildlife Manage 34:707–718
Kruskal JB (1964) Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29:1–27
Merritt RW, Cummins KW, Berg MB (2008) Aquatic insects of North America, 4th edn. Kendall Hunt Publishing, Dubuque
McCafferty WP (1983) Aquatic entomology: the fishermen's and ecologists' illustrated guide to insects and their relatives. Jones & Bartlett Learning, Burlington
McGoldrick JM, Nally M (1998) Impact of flowering on bird community dynamics in some central Victorian eucalypt forests. Ecol Res 13:125–139
McRae G, Camp DK, Lyons WG, Dix TL (1998) Relating benthic infaunal community structure to environmental variables in estuaries using nonmetric multidimensional scaling and similarity analysis. Environ Monit Assess 51:233–246
Morris DW (1996) Coexistence of specialist and generalist rodents via habitat selection. Ecology 77:2352–2352
Muenscher WC (1944) Aquatic plants of the United States. Comstock Publishing Company Inc., Cornell Univeristy, Binghamton
Nachtrieb JG (2008) The impact of invertebrates to four aquatic macrophytes: Potamogeton nodosus, P. illinoensis, Vallisneria americana, and Nymphaea Mexicana. Thesis, University of North Texas
Nachtrieb JG, Grodowitz MJ, Smart RM (2007) Impact of invertebrate herbivory on native aquatic macrophytes. Technical Report ERDC/TN APCRP-BC-9, U.S. Army Engineer WES, Vicksburg, MS
Neckles HA, Murkin HR, Cooper JA (2006) Influences of seasonal flooding on macroinvertebrate abundance in wetland habitats. Freshw Biol 23:311–322
Newman RM (1991) Herbivory and detritivory on freshwater macrophytes by invertebrates: a review. J North Am Benthol Soc 10:89–114
Owens CS, Smart RM, Dick GO (2008) Effects of water level fluctuation on Vallisneria americana Michx growth. J Aquat Plant Manage 46:117–119
Quinn GP, Keough MJ (2001) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Riis T, Biggs BJF (2003) Hydrologic and hydraulic control of macrophyte establishment and performance in streams. Limnol Oceanogr 48:1488–1497
Rybicki NB, Landwehr JM (2007) Long-term changes in abundance and diversity of macrophyte and waterfowl populations in an estuary with exotic macrophytes and improving water quality. Limnol Oceanogr 52:1195–1207
Santos R (1993) A multivariate study of biotic and abiotic relationships in a subtidal algal stand. Mar Ecol Prog Ser 94:181–190
Schad AN, Kennedy JH, Dick GO (2016) Secondary production and seasonal development of epiphytic Enallagma civile Hagen, 1861 (Odonata: Coenagrionidae) in a newly constructed urban wetland floodway ecosystem. Aquat Insect 73:159–173
Sher-Kaul S, Oertlie B, Castella E, Lachavanne JB (1995) Relationship between biomass and surface area of six submerged aquatic plant species. Aquat Bot 51:147–154
Shin PKS, Fong KYS (1999) Multiple discriminant analysis of marine sediment data. Mar Pollut Bull 39:285–294
Simpson EH (1949) Measurement of diversity. Nature 163:688
Smart RM, Dick GO (1999) Propagation and establishment of aquatic plants: a handbook for ecosystem restoration projects. Technical Report A-99-4, U.S. Army Engineer WES, Vicksburg, MS
Smart RM, Dick GO (2005) Update to the propagation and establishment of aquatic plants handbook. APCRP Miscellaneous Paper TR-05-4, U.S. Army ERDC, Vicksburg, MS
Smart RM, Doyle RD, Madsen JD, Dick GO (1996) Establishing native submersed aquatic plant communities in southern reservoirs. Technical Report A-96-2, U.S. Army Engineer WES, Vicksburg, MS
Smart RM, Dick GO, Doyle RD (1998) Techniques for establishing native aquatic plants. J Aquat Plant Manage 36:44–49
Smart RM, Dick GO, Snow JR, Honnell DR, Smith DH, Smith JK (2009) Ecological effects of exotic and native aquatic vegetation, ERDC/EL TR-09-10. U.S. Army ERDC, Vicksburg
Spieles DJ, Mitsch WJ (2000) Macroinvertebrate community structure in high- and low-nutrient constructed wetlands. Wetlands 20:716–729
Stuzenbaker CD (1999) Aquatic and wetland plants of the western gulf coast. TPWD Press, Austin
Thorp AG, Jones RC, Kelso DP (1997) A comparison of water-column macroinvertebrate communities in beds of differing submersed aquatic vegetation in the tidal freshwater Potomac River. Estuaries 20:86–95
Thorp JH, Covich AP (2010) Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego
Acknowledgements
This project was funded by the US Army Corps of Engineers Fort Worth District for biological monitoring of the Dallas Floodway Extension Lower Chain of Wetlands. We are indebted to Julie Nachtrieb, Nathan Harms, Dian Smith, Meredith Evans, Todd Sliger, David Holbrook, and Emily Yeoman for their help in the field and lab.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schad, A.N., Kennedy, J.H., Dick, G.O. et al. Aquatic macroinvertebrate richness and diversity associated with native submerged aquatic vegetation plantings increases in longer-managed and wetland-channeled effluent constructed urban wetlands. Wetlands Ecol Manage 28, 461–477 (2020). https://doi.org/10.1007/s11273-020-09724-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-020-09724-1