Abstract
The article presents the possibility of recovering nickel from waste produced as a result of wastewater neutralization during the electrochemical surface treatment of metals. Leaching the sludge with concentrated hydrochloric acid enabled the metals contained in the sludge from precipitate to the solution with the efficiency of 74.4% (Se) to 100% (Zn). The content of elements was determined using the ICP-OES method. The next step was the precipitation of metals from the obtained solution using various reagents. The precipitating reagents used were 0.5 M sodium hydroxide solution, 0.5 M sodium sulphide solution and 1% dimethylglyoxime solution. Selective precipitation made it possible to obtain nickel concentrates with the content of nickel ranging from 15.3 to 98.2% for the first two methods, whereas in the case of the third method based on a dimethylglyoxime solution, the obtained nickel concentrate purity was 94.3%. The process of leaching nickel-dimethylglyoxime complex (Ni-DMG) with sulphuric acid and crystallization enabled obtaining 99.4% purity nickel sulphate, which can be reused in the nickel plating of selected metals. The research shows that waste generated in the process of neutralization of wastewater from electroplating plants is a potentially important source of recycled nickel concentrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The development and security of the economy depend mainly on the possibility of obtaining raw materials. This problem is one of the key priorities for the functioning of the European Union. This was confirmed in the new industrial strategy for Europe published in 2020 (European Commission 2020).
It includes, among others, a list of 30 critical raw materials for the European Union. Nickel is not on the list, but a number of studies concerning the projected demand for this metal in the years to come draw attention to the rapid increase in demand for nickel. This is mainly due to the dynamic development of electric car production, which is related to the use of nickel in the production of electric energy storage batteries (www.pap.pl).
The process of electrochemical coating (Pawlikowski 1969) of objects made of materials characterized by low resistance to corrosion with layers of various metals, so as to improve their corrosion resistance and aesthetic values, was first used by J. Wright in 1840. In 1842, J. F. Böttger developed the method of electrolytic nickel plating.
Surface treatment of metals, associated with the electrochemical application of nickel coatings, generates a large amount of liquid and solid waste, which poses a threat to humans and the environment. As chemical processes generate wastewater containing a certain type of contaminants, it must be subjected to treatment and cannot be discharged directly into municipal wastewater or biological treatment plants due to the presence of copper(II) and nickel(II) ions. Wastewater from the electroplating industry contains different amounts of nickel. For example, the content of nickel in wastewater (Lekhlif et al., 2014; Sivaprakash et al., 2015) ranged from 16.3 to 300 mg/dm3.
For this reason, the treatment of wastewater from nickel plating processes is usually carried out in closed circuits, directly on the premises of the facility generating this waste.
The system of treatment of wastewater from the electroplating plant consists of a neutralization, ion exchange and special neutralization system.
As part of the special neutralization process, wastewater containing cyanides, nickel compounds contaminated with, among others, chrome, iron and copper is directed to the wastewater treatment tank, where it is subjected to treatment. The process of treatment includes, among others, neutralization of cyanides with sodium hypochlorite, chromium with sodium metabisulphite, heavy metals with coagulants and pH correction with NaOH and HCl. The waste suspension is directed to sedimentation tanks, from where it is supplied to filter presses for dewatering. The resulting sludge is stored in hazardous waste landfills. The problem of selective separation of nickel from electroplating waste is an important element of waste-free nickel circulation in the nickel plating process.
Papers (Claassen, 1966; Nechamkin, 1981) describe the method of selective nickel separation from iron and alloy steels.
Work (Brooks, 1985) describes a method of separating nickel compounds from wastewater sludge with the use of DMG as a precipitation reagent.
The application of extraction methods for the qualitative and quantitative separation of nickel and copper compounds is described in Blank et al. (1961).
Patent (Zhong, 2011) describes a method of purifying liquid nickel compounds by selective precipitation and crystallization methods.
Doctoral dissertation (Thomas, 2016) describes the method of nickel compounds selective precipitation using sodium trithiocarbonate (Na2CS3). Depending on the process conditions (pH), different contents of nickel ions precipitated from the tested solutions were obtained.
The aim of the work was to demonstrate the possibility of using waste generated in the processes of electrochemical metal surface treatment to obtain nickel concentrates. The use of this type of waste, which is currently landfilled and creates environmental problems, will reduce the use of natural resources, and thus improve the condition of the natural environment. The analysis of the literature shows that currently there are no solutions allowing for the low-waste and environmentally safe disposal of electroplating plant waste. The innovative solutions presented in the paper allow for the inclusion of electrochemical metal surface treatment processes in the closed-loop economy. The results of the work carried out are an important element of expanding knowledge in this area, and also present the possibilities of their practical implementation in industrial processes.
2 Material and Methods
2.1 Raw Materials
The subject of the research was the sludge produced as a result of neutralization of industrial wastewater from a metal surface treatment plant. The following were used in tests: nitric(V) acid (Chempur, analytical reagent grade), sulphuric(VI) acid (Chempur, analytical grade), hydrochloric acid (Stanlab, analytical grade), sodium sulphide nine-hydrate (Warchem, analytical grade), dimethylglyoxime (DMG) (AKTYN, analytical grade), ethyl alcohol 99% (Chempur, analytical grade), sodium hydroxide (POCH, analytical grade), certified multi-element solutions for ICP (AccuStandard, USA) with concentrations of analysed elements 100 mg/l and high-purity deionized water for dilutions (conductivity below 0.05 µS/cm, Direct-Q3 UV, Millipore).
2.2 Research Methodology
The sludge from the electroplating plant was dried to constant weight and subjected to diffraction tests (XRD) in order to determine the phase composition of the sample. The sludge was then mineralized in a solution of concentrated acids HNO3 and HCl in the 1:3 ratio. The obtained solution was suitably diluted and subjected to analysis using the ICP-OES method (Perkin Elmer, Optima 5300DV) (EN ISO 11885:2009) in order to determine the quantitative elemental composition of the tested material. For the next stage of the research, an agent for leaching metals from the sludge was selected, taking into account economic factors, such as concentrated hydrochloric acid. The obtained mixture was filtrated, and the filtrate was analysed in terms of Ni, Fe, Cr, Co, Cu, Mn, Al, Sn and Zn contents, using the ICP-OES method. The elemental composition of the tested wastewater sludge and the sludge subjected to concentrated HCl leaching is presented in Table 1. In further stages of the research, the solution obtained as a result of the treatment with concentrated HCl was divided and treated with precipitants.
In the first process, 20 ml of deionized water was added to 5 ml of the solution that had been previously subjected to leaching with a concentrated HCl solution; next, 0.5 M NaOH solution was added until the pH reached 6.2. The resulting mixture was filtered, and the filtrate was suitably diluted and prepared for analysis.
In the next step, a 1% solution of dimethylglyoxime (DMG) was added to the filtrate (H2O:ethyl alcohol, 3:1, v/v) to precipitate nickel(II) ions. The precipitate was separated from the solution through filtration, and the solution was subjected to analysis in terms of the quantitative composition of metals.
The pink precipitate of Ni-DMG complex (nickel(II)-dimethylglyoxime) obtained after adding 1% dimethylglyoxime solution was digested in diluted sulphuric(VI) acid so as to recover DMG and determine the content of the major component (Ni) and impurities.
In the second process, 0.5 M sodium sulphide (Na2S) solution was added to the solution prepared in the same way as in the first process until the pH reached 4.7. As a result of the process using sodium sulphide, black solid precipitate was produced. In the next step, the mixture was filtered and the solution prepared for further research. All analyses of the obtained aqueous solutions were conducted using the ICP-OES method.
2.3 Analytical Methods
Diffraction tests of the waste were performed using the powder method (DSH) in the Bragg–Brentano geometry using a D8 DISCOVER diffractometer, produced by Bruker, CuKα radiation, an Ni filter and a LYNXEYE_XE detector. The mineral composition was determined and calculated on the basis of licenced formulas in PDF-4 + 2020 RDB ICDD (International Centre for Diffraction Data) and databases: ICSD (Inorganic Crystal Structure Database) and NIST (National Institute of Standard and Technology). DIFFRAC v.4.2 and TOPAS v.4.2 Bruker AXS were used for registration and diagnostics.
The content of metals in aqueous solutions was determined using inductively coupled plasma optical emission spectrometry (EN ISO 11885:2009) (ICP-OES, Optima 5300DV, produced by Perkin Elmer, USA); the determination of metals in the obtained solutions was performed with the level of uncertainty of 15%, a coverage factor of 2 and a significance level of 95%, without taking into account the uncertainty related to sampling. An Inolab pH/ION/Cond 750 (manufactured by WTW, Germany) multi-parameter metre was used to measure the pH (EN ISO 10523:2012). The pH measurement was performed with an accuracy of ± 0.1 pH.
The grain morphology tests were carried out using a SU-3500 N scanning electron microscope (SEM) with variable vacuum by HITACHI, cooperating with an EDS UltraDry spectrometer produced by Thermo Scientific NORAN System 7, with the following parameters: accelerating voltage—15 keV, working distance (WD)—10 mm, pressure—30 Pa and vacuum—variable.
3 Results and Discussion
Based on the conducted XRD tests, the solid phase composition of the waste was calculated (Table 1).
The error analysis for individual mineral phases determined in the tested sample of waste from the electroplating plant has been given in Table 2.
Taking into account the error analysis, the ranges of components’ contents in the sample of waste from the electroplating plant were calculated (Table 3).
The determined crystalline phases of the waste from the electroplating plant are marked on the diffraction pattern (Fig. 1). High amount of the quartz (Q—on the diffraction pattern) in sludge is a result of using after electroplating process polishing materials that contained quartz. The presence of the remaining crystalline phases (Cr, Ni, Fe, Ca) is the result of the wastewater neutralization process with the use of wastewater treatment substances. A high content of heavy metals is present in the amorphous phase, which constitutes 46–47% of the sludge.
Figure 2 shows the image of the waste obtained by the SEM method.
The quantitative composition of the sludge was determined by digesting it in aqua regia and determining the concentration of individual elements in the obtained solution by the ICP-OES method. Table 4 shows the quantitative composition of the tested wastewater sludge and the efficiency of the process of leaching out individual metals from the sludge with concentrated hydrochloric acid.
The metals present in the highest concentrations (Cr, Cu, Fe, Ni, Zn) were leached into the solution with the efficiency reaching 94.4–100%
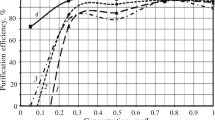
In the process in which the pH of the tested solution was raised to 6.2, most of the metals were precipitated. The obtained precipitate contained 15.3% nickel. A 1% alcoholic DMG solution was added to the remaining solution after prior filtration, which caused the removal of 98.5% of the nickel ions present in the solution. The addition of a precipitating agent in the form of 0.5 M Na2S solution to the solution (after digestion in concentrated HCl) resulted in the precipitation of black sediment, characteristic of block d metal sulphides. The addition of the sodium sulphide solution was completed when the pH reached 4.7. 99.7% of the nickel contained in the solution after wastewater sludge leaching with concentrated hydrochloric acid was precipitated. The contents of particular metals in the solutions after precipitation are presented in Table 5 and in the chart (Fig. 3).
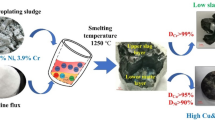
In order to obtain a nickel concentrate, the obtained sulphide sediment was digested in 1 M hydrochloric acid. The obtained solution contained mainly Cr, Cu, Fe and Zn ions and nickel sulphide (NiS) sediment. The sediment was washed with distilled water and, next, digested in concentrated (6 M) hydrochloric acid with an addition of a small amount of 3% hydrogen peroxide solution (H2O2). The determined concentration of nickel ions in the solution was 221 mg/dm3, which accounted for 86.7% of the original nickel content in the wastewater sludge.
A concentrated ammonia water solution was added to the obtained solution until an alkaline pH was reached. Next, a 1% alcoholic DMG solution was added. The obtained nickel-dimethylglyoxime complex was filtered off and subjected to further investigations.
The pink sludge constituting the Ni-DMG complex, which was obtained directly from the solution after digesting the wastewater sludge in concentrated hydrochloric acid, was 94.3% pure. The remaining 5.7% consisted of metal impurities which had not been washed from the sediment. The detailed composition of the tested substance is presented in Table 6.
Dimethylglyoxime (DMG) and nickel sulphate were separated from the Ni-DMG complex obtained by both the direct method (from concentrated hydrochloric acid) and the multi-stage method (using sodium sulphide and nickel sulphide sludge digested in concentrated hydrochloric acid). The method of leaching with sulphuric acid was used, as well as dissolution in sulphuric acid and recrystallization of dimethylglyoxime from the solution (Meenakshi et al., 2018). This operation resulted in obtaining crystalline nickel sulphate with a purity of 99.4%, which can be reused in the electrochemical nickel plating.
4 Conclusions
Sludge generated in the process of neutralization of wastewater from electroplating plants is a potentially important source of recycled nickel concentrates. Leaching the sludge produced in the process of neutralization of wastewater from a metal surface treatment plant with concentrated hydrochloric acid enabled the metals contained in the sludge from precipitate to the solution with the efficiency of 74.4% (Se) to 100% (Zn). In the case of nickel, the leaching efficiency reached 95.7%. The direct application of a 1% alcoholic dimethylglyoxime solution allowed for the selective separation of nickel compounds in the form of a pink precipitate, which was the Ni-DMG complex, with an efficiency of 98.5%. The resulting precipitate was 94.3% pure. The second method enabling separation of nickel compounds from the solution was based on 0.5 M sodium sulphide solution. As a result of the precipitation process, the nickel concentrate was separated in the form of nickel sulphide with a purity of 98.2%. The efficiency of the nickel separation process was 86.7% in relation to the content in the wastewater sludge. Digestion in concentrated hydrochloric acid and an addition of DMG allowed obtaining a concentrate with a nickel content of 97.2%. As a result of using the alkaline method (pH = 6.7) for metal precipitation from the solution obtained after digesting the precipitate in concentrated hydrochloric acid, a contaminated concentrate containing 15.3% of nickel was separated.
The research allowed concluding as follows:
-
1.
It is possible to obtain nickel concentrates with high efficiency (98.5%) by direct precipitation of the Ni-DMG complex from the solution after digesting the precipitate in concentrated hydrochloric acid. The degree of the obtained concentrate purity reached 94.3%.
-
2.
Application of the method of multi-stage precipitation of nickel compounds from wastewater sludge, with an intermediate stage of precipitation and digestion of nickel sulphide, allowed obtaining a nickel concentrate (in the form of nickel sulphide) with a purity of 98.2%. Compared to the method of direct separation of the concentrate, this process is characterized by a lower efficiency, amounting to 86.7% w/w of nickel compared to the content in the primary sludge.
-
3.
As it is impossible to eliminate co-precipitation of other metals, applying the method of direct precipitation of nickel compounds based on the alkalization of the solution after sludge digestion does not enable obtaining concentrates with a purity that would allow them to be reused in the processes of electrochemical nickel plating of metal surfaces.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- DMG:
-
Dimethylglyoxime
- EU:
-
European Union
- ICP:
-
Inductively coupled plasma
- ICP-OES:
-
Inductively coupled plasma optical emission spectrometry
- Ni-DMG:
-
Complex (nickel(II)-dimethylglyoxime)
- pH:
-
Logarithmic and inversely indicates the concentration of hydrogen ions in the solution
- SEM:
-
Scanning electron microscope
- T:
-
Temperature K (Kelvin scale)
- XRD:
-
X-ray diffraction
- XRF:
-
X-ray fluorescence
References
Blank, A. B., Bulgakova, A. M., Sizonenko, N. T., & Zhurnal, A. E. (1961). Successive extraction and photometric determination of traces of copper, nickel, iron, and manganese. Analiticheskoi Khimii, 16, 715–719.
Brooks C.S., (1985). Metal recovery from waste sludge’s Clyde Spear. Proceedings of the 39th Industrial Waste Conference, Glastonbury, CT, USA, 529–535
European Commission (2020). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Critical raw materials resilience: Charting a path towards greater security and sustainability. COM(2020) 474 final, Brussels, Belgium
Claassen, A. (1966). The determination of nickel with dimethylglyoxime in iron and steel containing cobalt and copper. The Analyst, 91, 725–731.
International Organization for Standardization, (2012). Water quality - Determination of pH, EN ISO 10523, Brussels, Belgium.
EN ISO 11885:2009, Water quality — Determination of selected elements by inductively coupled plasma optical emission spectrometry (ICP-OES)
Lekhlif, B., Oudrhiri, L., Zidane, F., Drogui, P., & Blais, J. F. (2014). Study of the electrocoagulation of electroplating industry wastewaters charged by nickel (II) and chromium (VI). Journal of Materials and Environmental Science, 5(1), 111–120.
Meenakshi, R., Laxmi, P. B., Barsha, D., Abdul, S., & Kali, S. (2018). Recovery of dimethylglyoxime (DMG) from Ni-DMG complexes. Hydrometallurgy, 176, 229–234. https://doi.org/10.1016/j.hydromet.2018.01.014
Nechamkin L., (1981). The recovery of nickel from stainless steel. Howard Chem. News 13, No124, 6.
Pawlikowski, P. (1969) Zarys Elektrochemii Technicznej, Wydawnictwo Naukowo-Techniczne, Warsaw, Poland.
Sivaprakash K., Blessi A., Madhavan J., (2015), Biosorption of nickel from industrial wastewater using Zygnema sp., Journal of The Institution of Engineers (India), Series A, pp 319–326, Vol. 96, 4. https://doi.org/10.1007/s40030-015-0131-1
Thomas, M.G., (2016). Optimization of the process of removing selected heavy metals and organic compounds from wastewater from the production of printed circuits. Doctoral dissertation, GIG. https://www.pap.pl/aktualnosci/news%2C711612%2Cke-nowa-lista-surowcow-krytycznych-nowa-strategia-zabezpieczenia-dostawAccessed date 05.06.2020
Zhong Q. Z., (2011). Method for purification and impurity removal of nickel liquid, Patent CN101603125B.
Funding
This work was supported by the Ministry of Science and Higher Education, Republic of Poland (Statutory Activity of the Central Mining Institute, Task no. 11182010–335).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Świnder, H., Lejwoda, P. Obtaining Nickel Concentrates from Sludge Produced in the Process of Electrochemical Metal Surface Treatment. Water Air Soil Pollut 232, 505 (2021). https://doi.org/10.1007/s11270-021-05456-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05456-x