Abstract
Wastewater reuse coupled to managed aquifer recharge (MAR) provides a means to store and reuse treated wastewater (TWW) year-round. Determining the fate of nutrients in the subsurface during MAR remains challenging for environmental regulation due to the interaction of the MAR source water with site specific aquifer conditions. To facilitate the understanding of natural treatment processes, this study uses operational monitoring data from a full-scale aquifer storage and recovery (ASR) scheme using TWW to assess nutrient (N and P) transformation and fate. Analysis of median water quality injected into and recovered from the ASR wells for two complete ASR cycles (June 2014 to March 2016) was used to describe the removal of nutrients in an anoxic carbonate aquifer. Total nitrogen (TN) removal was dominated by redox processes, with median removal of 40 to 60% for TN and nitrate (the dominant N species) and higher removal of ammonia (95%) and total Kjeldahl nitrogen (TKN) (70%). Total phosphorous (TP) removal was also observed (~ 90%) due to sorption (filterable reactive phosphorous median removal of ~ 80%). A 40% increase in median salinity was evident within each ASR cycle due to recovery of the entire volume of injected water each year (ambient groundwater is 200% higher in TDS, on average). A reduction in salinity of the recovered water could be achieved by leaving a residual of source water in the aquifer to create a buffer zone between the ambient groundwater and the fresher source water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Wastewater reuse coupled to managed aquifer recharge (MAR) provides a means to store and reuse treated wastewater (TWW) for agricultural production throughout the entire year. MAR takes advantage of suitable aquifers to provide storage, which is important for security of supply and to balance the supply of wet season runoff with dry season irrigation demand (Dillon et al., 2008). Aquifer storage and recovery (ASR) is a MAR technique that uses the same well for injection into and recovery from a confined aquifer (Pyne, 2006), which has been adopted extensively in the USA for storage of drinking water (Pyne, 2006) and in Australia for storage and use of urban stormwater (e.g. Page et al., 2015) and treated wastewater (e.g. Vanderzalm et al., 2013). ASR is often used to store high-quality water in brackish aquifers (Pyne, 2006) and requires creation of a buffer zone to separate the stored water from the surrounding brackish, ambient groundwater. The buffer zone is typically created by leaving a residual of the injectant in the aquifer and may be done prior to commencement of the ASR cycles, and/or in between ASR cycles (Pyne, 2006).
TWW represents a concentrated but reasonably uniform quality and quantity resource whose impact on receiving environments requires management. Typically, most wastewater treatment plants are specifically designed to remove nutrients such as nitrogen and phosphorus prior to aquatic discharge as nitrogen and phosphorus are associated with eutrophication and water quality issues in receiving environments (Collins et al., 2010). In response to this, environmental regulations have evolved and generally require higher levels of treatment, especially nutrient removal prior to discharge. To date these regulations have not considered that in addition to providing storage, aquifers can serve as a treatment barrier providing improvements in water quality. For example, Dillon et al. (2008) reported nutrient and organic carbon removal from treated wastewater (Fox, 2002; Kim et al., 2004; Sharma et al., 2011; Abel et al., 2012; Vanderzalm et al., 2006, 2013, 2018) along with various other potential contaminants such as pathogens (Sidhu et al., 2015; Page et al., 2017), suspended solids and turbidity (Page et al., 2015) and trace organic chemicals (Patterson et al., 2011; Shareef et al., 2013).
Yet, to date the aquifer treatment is not considered as a management option for nutrients (N, P) in TWW as performance remains poorly quantified. As a direct result, environmental legislation and management remains onerous but justifiably cautious in the absence of enough robust long-term data. For example, legislative requirements in some Australian jurisdictions require that all recharged TWW, volumetrically at least, be recovered annually. This leaves no opportunity for inter-season water banking, freshening of naturally brackish aquifers and creates poorer quality recovered water when brackish groundwater is also extracted into the water reuse supply system.
To facilitate the understanding of natural treatment processes, this study uses operational monitoring data from a full-scale ASR scheme using TWW to assess nutrient (N and P) transformation and fate. The specific aims of this study were the following: (i) to calculate the removal of nitrogen and phosphorus at a TWW full-scale ASR site used for irrigation; (ii) assess the treatment performance of the aquifer as a treatment barrier; (iii) compare the water quality after an activated sludge-ASR treatment train to a membrane bioreactor with respect to protection of a downstream environmental receptor; and (iv) recommend operational measures to maximise the benefit of water recycling via aquifers and also protect the environment and human health.
2 Material and Methods
The Willunga Basin Water Recycling Scheme based in South Australia, Australia, commenced in 1999 in response to the Millennium drought, irrigation water scarcity and increasing regulation to manage groundwater use. Today, the customer base has now grown to > 180 irrigators, with > 120 km of pipeline supplying TWW for drip irrigation of high economic value vineyards (ISF, 2013; SA Water, 2013). The TWW used for irrigation is sourced from the Christies Beach wastewater treatment plant (WWTP).
The Christies Beach WWTP receives ~ 30,000 m3/d from mainly a residential urban sewage catchment (SA Water, 2013). The WWTP consists of two activated sludge treatment trains: A/B-plant uses the Modified Ludzak-Ettinger (MLE) process and C-plant uses the membrane bioreactor process (MBR) process. The A/B-plant consists of primary treatment, secondary treatment via MLE, clarification and chlorine disinfection. TWW from A/B-plant is approved for irrigation use and must meet the following regulatory values: biological oxygen demand (BOD) < 20 mg/L, total suspended solids (TSS) < 30 mg/L and Escherichia coli < 1000 colony forming units (cfu)/100 mL (annual median). The C-plant consists of 2 mm screening, MBR and UV disinfection and was commissioned in 2012 for improved nutrient removal prior to discharge to the marine environment, the Gulf of St Vincent.
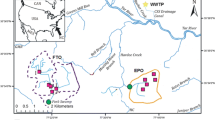
The Aldinga ASR scheme was established to store TWW, which would normally be discharged to the Gulf of St Vincent (Fig. 1). The ASR scheme is located adjacent to the Aldinga WWTP and recycled water treatment plant (RWTP), approximately 18 km to the south of the Christies Beach WWTP. The TWW from the Christies Beach WWTP is injected into a confined limestone aquifer within the Tertiary Port Willunga Formation at a target interval of 30–70 m below ground during winter. The entire injected TWW volume is subsequently recovered in summer for irrigation, via four production bores. The MAR component of the scheme was commissioned in 2010 and is currently approved by the EPA South Australia for injection and recovery of 400,000 m3/year.
The Aldinga aquifer storage and recovery (ASR) scheme showing four ASR wells (6527–1770, 6527–1771, 6527–1772, 6527–1811). The ASR scheme receives treated wastewater (TWW) from Christies Beach wastewater treatment plant (WWTP) (not shown, ~ 18 km to the north) and is located adjacent to Aldinga WWTP and recycled water treatment plant (RWTP). Ambient groundwater quality is from observation well no. 6527–1052, sampled July 2008 prior to commencement of ASR. See Table 1 for ASR well details
TWW quality is routinely monitored at the end of the Christies Beach WWTP A/B-plant post chlorine disinfection and post UV disinfection at C-plant. As the Aldinga ASR scheme is intended for storage when irrigation water is not required, it follows that the source water for use in ASR originates from A/B-plant. The treated wastewater water is then injected into the target aquifer and recovered via inlet and outlet pipe manifolds. Recovered water quality is monitored by an automatic sampler and grab samples on the common outlet manifold and represents the water quality of any of the four production bores. Individual bores can be manually sampled via the sample tap at each of the bores.
Water quality parameters are determined by the NATA accredited Australian Water Quality Centre, Adelaide, South Australia, and in accordance with Standard Methods (APHA, 2005). The nutrients nitrogen and phosphorus (N, P) and salinity (total dissolved solids, TDS) are reported in this current study.
Water quality data for two complete ASR cycles during the period of June 23, 2014 to March 22, 2016 for TWW (input) and ASR recovered water (output) were used. During this interval 530,000 m3 of TWW were injected and 533,000 m3 recovered (Fig. 2). Prior to this, three ASR cycles with smaller injection volumes (< 60,000 m3) had been undertaken between 2012 and 2014. The comparison of input and output assesses nutrient removal during ASR, where the same well is used for injection and recovery. In these ASR cycles, the input originated from the A/B-plant, meaning the TWW had been treated by the MLE process. The C-plant, employing MBR treatment, is intended to provide greater protection to the marine environment through greater removal of nutrients. The water quality recovered from ASR is compared to the quality of TWW treated by MBR, to determine if ASR can provide adequate treatment prior to marine discharge.
The 5th, 50th (median) and 95th percentile water quality data were used to describe the TWW and ASR recovered water. The efficiency of natural treatment was calculated from changes in the median water quality, comparing ASR recovered water (ASR Out) to that after MLE treatment (ASR In) for each of the two ASR cycles.
The water recovered from an ASR well is a mixture of source water and ambient groundwater. The mixing fraction of the injected stormwater present in recovered water samples, f, was estimated by the method described by following equation (Pavelic et al. 2006) (Eq. 1). Chloride is typically used in this calculation as it is a conservative solute within the aquifer, assuming the injected concentration is stable and comparable to the average concentration. Chloride data was not available, so the mixing fraction was estimated with TDS, recognising that TDS comprises of both conservative and nonconservative solutes. However, the high TDS concentrations of the end-members reduce the significance of changes due to geochemical reaction processes.
where:
- [TDS]recovered:
TDS in the recovered water
- [TDS]ambient:
TDS in ambient groundwater; based on median value
- [TDS]injected:
TDS in injected water; based on median value
3 Results and Discussion
TWW was stored in a confined carbonate aquifer via an ASR scheme during the wet season (winter) for recovery and non-potable use in the dry season (summer). A summary of the ASR injectant and recovered water quality data and available ambient groundwater quality data is provided in Table 2 and Table 3.
The ambient groundwater in the storage zone, the T2 aquifer, is brackish with TDS of ~ 2200 mg/L, anoxic (< 0.5 mg/L dissolved oxygen) and low in nutrients (Table 3). In contrast for the two ASR cycles, the TWW was fresher (median TDS 680–730 mg/L), oxygenated and rich in nitrogen (median TN 14–16 mg/L) and phosphorus (median TP 8.5–8.6 mg/L). The ASR recovered water had a ~ 40% higher median TDS than that injected (TDS 1000–1100 mg/L; Fig. 3) and was typically lower in nutrients than that injected with median nitrogen TN 5.9–8.4 mg/L and TP 1.3 mg/L.
Summary of TDS injected into (after Modified Ludzak-Ettinger (MLE) process) and recovered from aquifer storage and recovery (ASR) (MLE and ASR treatment) for two aquifer storage and ASR cycles. Box plot indicates median (line within box), 25th and 75th percentile values (box boundary), 10th and 90th percentile values (whiskers) and 5th and 95th percentile values (closed circles) and dotted lines indicate ambient groundwater and drinking water guideline
Based on median water quality, the water recovered from ASR is a mixture of TWW and ambient groundwater, and this mixing between two water sources leads to higher salinity in the recovered water than that injected. This same mixing can contribute to apparent nutrient removal through dilution. A conservative tracer, such as chloride, is typically used to quantify the fraction of mixing between two water sources (Eq. 1). Subsequently the role in reduction of nutrient concentration of dilution or hydrogeochemical reaction can be quantified. In the absence of a suitable conservative tracer, TDS was used to estimate the fraction of TWW within recovered water; ~ 70–80% on average (Table 4 and Fig. 2). Notably, recovery of the entire volume of injected TWW results in recovery of > 40% of the higher salinity groundwater toward the end of the recovery period. Recovery of this brackish ambient groundwater could be minimised by creation of a buffer zone that separates inter-recovery period stored water from the surrounding ambient groundwater (Pyne, 2006). Creation and maintenance of this buffer zone requires some residual TWW to remain in the aquifer in between ASR cycles. Typically, recovery from an ASR scheme would cease when a trigger value for TDS is reached. However, the current Aldinga ASR operation licence prevents creation of this buffer zone or the use of TDS to trigger the end of recovery, due to the requirement to recover all TWW injectant and higher salinity ASR recovered water. While TDS may not be conservative and gives only an approximation of the mixing between injectant and ambient groundwater, it is useful to consider if dilution can explain the observed nutrient removal. Based on median concentrations, 30% dilution with ambient groundwater would result in TN in the recovered water of ~ 10–11 mg/L and TP of ~ 6–7 mg/L. The measured concentrations were considerably lower, with a reduction of > 3 mg/L TN and 5 mg/L TP, indicative of removal via natural treatment processes.
TN (Fig. 4) and TP (Fig. 5) concentrations in ASR recovered water are reduced in comparison to the injectant (after MLE treatment). Median TN is 14–16 mg/L in the TWW injectant and 6–8 mg/L in the ASR recovered water. This TN is predominantly present as oxidised species, nitrate and nitrite with median concentrations of 12–13 mg/L injected and 5–8 mg/L recovered. Despite removal of at least 40% TN within the aquifer, the median nitrogen concentrations after ASR remain 1–3 mg/L (20–40%) greater than after MBR treatment, which results in a median TN of 5 mg/L and NOx-N of 4 mg/L. Neither treatment train provides sufficient N removal without additional dilution to meet the ambient groundwater TN concentration of 1.6 mg/L, or the more stringent local marine ecosystem guideline of 0.25 mg/L for the 90th percentile value (EPA, 2013) (Fig. 4).
Summary of TN and NOx-N injected into (Modified Ludzak-Ettinger (MLE) treatment) and recovered from aquifer storage and recovery (ASR) (MLE and ASR treatment) in comparison to TN and NOx-N after membrane bioreactor (MBR) treatment for a ASR cycle A and b ASR cycle B. Box plot indicates median (line within box), 25th and 75th percentile values (box boundary), 10th and 90th percentile values (whiskers) and 5th and 95th percentile values (closed circles) and dotted lines indicate ambient groundwater and guideline or trigger values. LTV = long-term trigger value for use up to 100 years, STV = short-term trigger value for use up to 20 years (ANZECC, 2000)
Summary of TP and FRP injected into (Modified Ludzak-Ettinger (MLE) treatment) and recovered from aquifer storage and recovery (ASR) (MLE and ASR treatment) in comparison to TP and FRP after membrane bioreactor (MBR) treatment for a ASR cycle A and b ASR cycle B. Box plot indicates median (line within box), 25th and 75th percentile values (box boundary), 10th and 90th percentile values (whiskers) and 5th and 95th percentile values (closed circles) and dotted lines indicate ambient groundwater and guideline or trigger values. LTV = long-term trigger value for use up to 100 years, STV = short-term trigger value for use up to 20 years (ANZECC, 2000)
Injectant and recovered TN concentrations and the effectiveness of natural treatment vary between individual ASR cycles (Table 2). TN concentrations in the injectant ranged between 13 and 26 mg/L in cycle A and between 12 and 16 mg/L in cycle B, compared with 4.3 to 7.4 mg/L in the recovered water in cycle A and 6.5 to 10 mg/L in cycle B. TN in the injectant is less variable in cycle B (coefficient of variation (CV) 8%) than A (CV 23%) (Table 2), though removal in the subsurface is apparently greater in cycle A. This behaviour is repeated for NOx-N, the dominant nitrogen species. NOx-N concentrations in the injectant varied between 9.8 and 22 mg/L in the injectant in cycle A and 11 and 15 mg/L in cycle B, with 2.6 to 7.0 mg/L in the recovered water in cycle A and 4.9 to 9.3 mg/L in cycle B (Table 2). Ammonia-N concentrations in the injectant were between < 0.5 to 2.5 mg/L in the TWW injectant and < 0.005 to 0.75 mg/L in the ASR recovered water. The removal for TN and NOx-N was ~ 60% in cycle A and ~ 40% in cycle B, which includes both removal and transformation of nitrogen species. Median removal of 66 to 85% for TKN and > 95% for ammonia indicates that both organic nitrogen and ammonia are removed through mineralisation of organic nitrogen to ammonium and then subsequent oxidation. This contrasts with 35 to 60% removal of NOx-N. The calculated removal does not consider the removal of nitrate formed in situ via oxidation. However, the net loss of TN represents nitrification of ammonium to form nitrate (Eq. 2), which is subsequently converted to nitrogen gas through denitrification (Eq. 3). Some of the converted nitrate is subsequently recovered prior to denitrification being complete. Nitrification and the subsequent denitrification are biologically mediated redox transformations. The opportunity for nitrification is limited to immediately after injection where oxic conditions may persist, following the injection of oxygenated TWW into an anoxic aquifer. Denitrification then prevails as the key TN removal mechanism but is limited by the availability of biodegradable dissolved organic carbon (BDOC) to facilitate this kinetic reaction (Vanderzalm et al., 2013, 2018). This BDOC is sourced from the influent TWW as the T2 aquifer is low in organic carbon (< 0.5%, Vanderzalm et al., 2010). The difference in NOx-N removal between cycles cannot be explained with the available data but is likely to be influenced by the availability of BDOC in TWW. BDOC data was not available. TWW dissolved organic carbon (DOC) was not quantified in cycle A and the median concentration was 9.7 mg/L in cycle B. DOC was not measured in recovered water, so removal during ASR cannot be quantified. Assuming all TWW DOC was consumed by denitrification (Eq. 3) can explain up to 9 mg/L NO3-N removal. TN removal of 5.6 mg/L observed in cycle B (Table 2) can be explained by dilution and ~ 2 mg/L removal via denitrification (Eq. 2). This suggests approximately 20% of TWW DOC is biodegradable, as previously reported (Vanderzalm et al., 2006). This suggests microbially mediated denitrification is a sustainable removal process for TN in TWW ASR in an anoxic aquifer but relies on the presence of enough BDOC in the injectant (TWW) to stimulate this process.
TP concentrations are almost equivalent to filterable reactive phosphorus (FRP) concentrations, so only TP is presented and discussed. Median TP was reduced from 8.6 mg/L in the TWW injectant to 1 mg/L in the ASR recovered water. In this instance the aquifer provides greater removal of P than the MBR treatment process, with median TP of 5 mg/L after MBR (Fig. 5). As with N, neither treatment train provides sufficient P removal to meet the ambient groundwater TP concentration of 0.013 mg/L, or the marine ecosystem guideline of 0.025 mg/L for the 90th percentile value (EPA, 2013).
TP concentrations and removal due to natural treatment are more consistent in the two ASR cycles, than observed for TN. TP in the injectant ranged between 6.5 and 10 mg/L in cycle A and between 7.5 and 9.8 mg/L in cycle B. Recovered TP varied from 0.94 to 2.6 mg/L in cycle A and from 1.1 to 3.2 mg/L in cycle B (Fig. 5). The median removal of TP was ~ 90% and FRP was ~ 80% (Fig. 6); considerably higher than previously reported for TWW ASR (Vanderzalm et al., 2013). Vanderzalm et al. (2013) reported that only injected TP concentrations > 1 mg/L were consistently removed by adsorption during TWW ASR where the injectant TP ranged between 0.05 and 6 mg/L. In the previous case, sorption was considered to have a limited capacity and could be reversed. These limitations for P removal by sorption were not evident for this study’s ASR scheme over multiple years of operation, despite introducing considerably higher TP loadings to the aquifer. The superior sorption performance may be explained by redox conditions within the storage zone. In the current Aldinga ASR scheme nitrate is not completely consumed in the aquifer and therefore sorption sites on amorphous iron hydroxide and iron oxide coatings on carbonate mineral surfaces are retained. In contrast Vanderzalm et al. (2013) reported iron reducing conditions and some loss of sorption sites during the storage phase. This demonstrates that (for some ASR schemes at least) there is considerable potential to harness natural treatment in an aquifer as a potential alternative to engineered treatments such as MBR.
4 Conclusion
Treated wastewater injected, stored and recovered via an ASR scheme operated in an anoxic, carbonate aquifer reported a median removal of 40 to 60% for TN and NOx-N, the dominant N species over two ASR cycles, with higher removal of TKN (> 70%) and NH4-N (> 95%). The removal was attributed to nitrification of ammonium to form nitrate and subsequent denitrification to produce nitrogen gas. Median TP removal due to sorption was consistent at ~ 90% over the two ASR cycles. The efficiency of treatment for TP was more effective than previously observed for TWW ASR, presumably due to the stability or iron oxide sorption sites.
The MLE process combined with ASR provides greater P removal than the MBR treatment step alone. However, MBR provides greater N removal. Therefore, the choice of treatment train must be informed by the requirements for protection of downstream environmental receptors. Neither treatment train can meet the stringent marine quality guidelines or ambient groundwater concentration of N and P.
In the current study, the requirement to recover all the injected TWW each year leads to a ~ 40% median increase in the salinity of the ASR recovered water. This environmental constraint could potentially be eased with the establishment of a buffer zone. This requires a residual of TWW injectant in the aquifer but would reduce the extent of mixing between the injectant and the brackish ambient groundwater, thus lowering recovered water TDS. Salinity is typically the key constraint for uptake of TWW for irrigation. Thus, lowering the salinity of the recovered water may increase uptake and subsequently reduce the remaining volume of treated wastewater requiring coastal discharge.
References
Abel, D. T. A., Sharma, S. K., Malolo, Y. N., Maeng, S. K., Kennedy, M. D., & Amy, G. L. (2012). Attenuation of bulk organic matter, nutrients (N and P), and pathogen indicators during soil passage: Effect of temperature and redox conditions in simulated soil aquifer treatment (SAT). Water, Air, and Soil Pollution, 223, 5205–5220.
APHA (American Public Health Association). (2005). Standard methods for the examination of water and wastewater (735 pp). Washington: APHA-WEF-AWWA.
Collins, K. A., Lawrence, T. J., Stander, E. K., Jontos, R. J., Kaushal, S. S., Newcomer, T. A., Grimm, N. B., & Ekberg, M. L. C. (2010). Opportunities and challenges for managing nitrogen in urban stormwater: A review and synthesis. Ecological Engineering, 36, 1507–1519.
Dillon, P., Page, D., Vanderzalm, J., Pavelic, P., Toze, S., Bekele, E., Sidhu, J., Prommer, H., Higginson, S., Regel, R., Rinck-Pfeiffer, S., Purdie, M., Pitman, C., & Wintgens, T. (2008). A critical evaluation of combined engineered and aquifer treatment systems in water recycling. Water Science and Technology, 57(5), 753–762.
EPA. (2013). Adelaide coastal waters quality improvement plan (ACWQIP). South Australia: Environmental Protection Authority.
Fox, P. (2002). Soil aquifer treatment: an assessment of sustainability. In P. J. Dillon (Ed.), Management of Aquifer Recharge for Sustainability, Proceedings 4thInternational Symposium on Artificial Recharge of Groundwater, Adelaide, South Australia, 22–26 September 2002 (pp. 107–110). Lisse: Swets & Zeitlinger.
Institute for Sustainable Futures (ISF): 2013, Willunga Basin case study; building industry capability to make recycled water investment decisions. Prepared by the Institute for Sustainable Futures, University Sydney for the Australian Water Recycling Centre of Excellence. http://waterrecyclinginvestment.com/wp-content/uploads/2013/11/ISF019_AWRC_D8_Willunga_4-2.pdf
Kim, J.-W., Kim, J., Choi, H., & Schwartz, F. W. (2004). Modelling the fate and transport of organic and nitrogen species in soil aquifer treatment process. Water Science and Technology, 50(2), 255–261.
NHMRC-NRMMC: 2011, Australian drinking water guidelines. Paper 6. National Water Quality Management Strategy (version 3.2 updated February 2016), National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra. https://www.nhmrc.gov.au/_files_nhmrc/file/publications/nhmrc_adwg_6_february_2016.pdf
Page, D. W., Peeters, L., Vanderzalm, J., Barry, K., & Gonzalez, D. (2017). Effect of aquifer storage and recovery (ASR) on recovered stormwater quality variability. Water Research, 117, 1–8.
Page, D. W., Vanderzalm, J. L., Barry, K. E., Torkzaban, S., Gonzalez, D., & Dillon, P. J. (2015). E. coli and turbidity attenuation during urban stormwater recycling via aquifer storage and recovery in a brackish limestone aquifer. Ecological Engineering, 84, 427–434.
Patterson, B. M., Shackleton, M., Furness, A. J., Bekele, E., Pearce, J., Linge, K. L., Busetti, F., Spadek, T., & Toze, S. (2011). Behaviour and fate of nine recycled water trace organics during managed aquifer recharge in an aerobic aquifer. Journal of Contaminant Hydrology, 112, 53–62.
Pavelic, P., Dillon, P. J., & Simmons, C. T. (2006). Multiscale characterization of a heterogeneous aquifer using an ASR operation. Ground Water, 44(2), 155–164.
Pyne, R. D. G. (2006). Groundwater recharge and Wells: A guide to aquifer storage recovery (2nd ed.). CRC Press.
SA Water. (2013). SA Water Regulatory Business Proposal 2013: Attachment E.2 SA Water Wastewater treatment plants and catchments. Adelaide: Government of South Australia.
Shareef, A., Page, D., Vanderzalm, J., Williams, M., Gupta, V., Dillon, P., & Kookana, R. (2013). Biodegradation of two herbicides simazine and diuron during managed aquifer recharge of stormwater. Clean: Soil, Air, Water, 42(6), 745–752.
Sidhu, J. P. S., Toze, S., Hodgers, L., Barry, K., Page, D., Li, Y., & Dillon, P. (2015). Pathogen decay during managed aquifer recharge at four different sites with different geochemical characteristics and recharge water sources. Journal of Environmental Quality, 44, 1402–1412.
Sharma, S. K., Hussen, M., & Amy, G. (2011). Soil aquifer treatment using advance primary effluent. Water Science and Technology, 64(3), 640–646.
Vanderzalm, J. L., Page, D. W., Barry, K. E., & Dillon, P. J. (2010). A comparison of the geochemical response to different managed aquifer recharge operations for injection of urban storm water in a carbonate aquifer. Applied Geochemistry, 25, 1350–1360.
Vanderzalm, J. L., Page, D. W., Barry, K. E., & Dillon, P. J. (2013). Application of a probabilistic modelling approach for evaluation of nitrogen, phosphorus and organic carbon removal efficiency during four successive cycles of aquifer storage and recovery (ASR) in an anoxic carbonate aquifer. Water Research, 47, 2177–2189.
Vanderzalm, J. L., Page, D. W., Dillon, P. J., Barry, K. E., & Gonzalez, D. (2018). Nutrient removal during stormwater aquifer storage and recovery in an anoxic carbonate aquifer. Journal of Environmental Quality, 47(1), 276–286. https://doi.org/10.2134/jeq2016.12.0486.
Vanderzalm, J. L., Le Gal La Salle, C., & Dillon, P. J. (2006). Fate of organic matter during aquifer storage and recovery (ASR) of reclaimed water in a carbonate aquifer. Applied Geochemistry, 21, 1204–1215.
Acknowledgements
The authors gratefully acknowledge the thoughtful review comments provided by two anonymous reviewers and Dr. Jason Belden, Associate Editor Water, Air, & Soil Pollution, along with Dr. Anna Kaksonen and Dr. Elise Bekele, both from CSIRO, to improve the quality of this manuscript. The authors thank Adam James from SA Water for his helpful discussions during a site visit to the Aldinga WWTP in 2016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vanderzalm, J., Page, D., Regel, R. et al. Nutrient Transformation and Removal from Treated Wastewater Recycled Via Aquifer Storage and Recovery (ASR) in a Carbonate Aquifer. Water Air Soil Pollut 231, 65 (2020). https://doi.org/10.1007/s11270-020-4429-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-4429-x