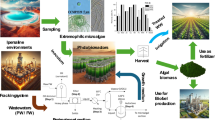
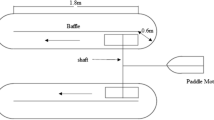
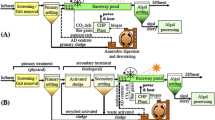
Abstract
Hydraulic fracturing is used to enhance oil and gas extraction from tight shale formations and generates millions of gallons of wastewater which needs to be cleaned up prior to disposal or reuse. The current technologies used for the management of this wastewater present technical, economic, and environmental challenges. Hence, the main objective of this study was to examine the potential of algal remediation of hydraulic fracturing wastewater (PW) as an alternative method. Considering that PW contains very low concentration of the nutrients needed for algae growth PW supplemented with animal wastewater (AW-PW) was also examined. Biomass production capacity, average biomass productivity, and specific growth rate of the microalgae strain used in the study, Picochlorum oklahomensis, were 1.87 g L−1, 268 mg L−1 day−1, and 0.35 day−1, respectively, when grown in PW. Complete nitrate, ammonia, and phosphate removal could be achieved by growing algae in PW. Supplementation of PW with animal wastewater enhanced biomass production (1.87–2.40 g L−1) and lipid content (15–25% wt) in the produced algal biomass. A mathematical model with a correlation coefficient of greater than 0.94 was developed to describe the growth kinetics of algae grown in AW-PW.




Similar content being viewed by others
References
Acharya, H. R., Henderson, C., Matis, H. Kommepalli, H., Moore, B., Wang, H. (2011). Cost effective recovery of low-TDS frac flowback water for re-use. Final Report. Resource document. Department of Energy. https://netl.doe.gov/sites/default/files/2018-03/FE0000784_FinalReport.pdf. Accessed 9 April 2020.
American Public Health Association-APHA. (2005). Standard methods for the examination of water and wastewater 21th edition. In: American public health association (APHA), American Water Works Association, Water Pollution Control Federation, pp. 2–48.
Ammar, S. H., Khadim, H. J., & Mohamed, A. I. (2018). Cultivation of Nannochloropsis oculata and Isochrysis galbana microalgae in produced water for bioremediation and biomass production. Environmental Technology and Innovation, 10, 132–142.
Annan, J. N. (2008). Growth response of the green alga Picochlorum oklahomensis to nutrient limitation and slainity stress. (PhD dissertation), Oklahoma State University, Stillwater, OK.
Arriada, A. A., & Abreu, P. C. (2014). Nannochloropsis oculata growth in produced water: an alternative for massive microalgae biomass production. Brazilian Journal of Petroleum and Gas, 8(3), 119–125.
Arthur, J. D., Langhus, B. G., Patel, C. (2017). Technical summary of oil and gas produced water treatment technologies. http://www.all-llc.com/publicdownloads/ALLConsulting-WaterTreatmentOptionsReport.pdf. Accessed on 18 October 2017.
Banks, H. T., Collins, E., Flores, K., Pershad, P., Stemkovski, M., & Stephenson, L. (2017). Statistical error model comparison for logistic growth of green algae (Raphidocelis subcapitata). Applied Mathematics Letters, 64, 213–222.
Barrera Bernal, C., Várquez, G., Barceló Quintal, I., & Bussy, A. L. (2008). Microalgal dynamics in batch reactors for municipal wastewater tretament containing deiry sewage water. Water, Air, Soil and Pollution, 190, 259–270.
Benko, K. L., & Drewes, J. E. (2008). Produced water in the Western United States: geographical distribution, occurrence, and composition. Environmental Engineering Science, 25(2), 239–246.
Bhola, V. K., Swalaha, F. M., Nasr, M., Kumari, S., & Bux, F. (2016). Physiological responses of carbon-sequestering microalgae to elevated carbon regimes. European Journal of Phycology, 51(2016), 401–412.
Bonilla, I., Garcia-González, M., & Mateo, P. (1990). Boron requirement in cyanobacteria. Its possible role in the early evolution of photosynthetic organisms. Plant Physiology, 94(4), 1554–1560.
Brdar-Jakanović, M. (2020). Boron toxicity and deficiency in agricultural plants. International Journal of Molecular Sciences, 21, 1424.
Chaiwong, K., Kiatsiriroat, T., Vorayos, N., & Thararax, C. (2013). Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass and Bioenergy, 56, 600–606.
Chen, C., Ma, X., & He, Y. (2012). Co-pyrolysis characteristics of microalgae Chlorella vulgaris and coal through TGA. Bioresource Technology, 117, 264–273.
Cluff, M. A., Hartsock, A. M., MacRae, J. D., Carter, K., & Mouser, P. J. (2014). Temporal changes in microbial ecology and geochemistry in produced water from hydraulically fractured Marcellus shale gas wells. Environmental Science and Technology, 48(11), 6508–6517.
Concas, A., Pisu, M., & Cao, G. (2013). Mathematical modelling of Chlorella vulgaris growth in semi-batch photobioreactors fed with pure CO2. Chemical Engineering Transactions, 32, 1021–1026.
Concas, A., Pisu, M., & Cao, G. (2016a). A novel mathematical model to simulate the size-structured growth of microalgae strains dividing by multiple fission. Chemical Engineering Journal, 287, 252–268.
Concas, A., Malavasi, V., Costelli, C., Fadda, P., Pisu, M., & Cao, G. (2016b). Autotrophic growth and lipid production of Chlorella sorokiniana in lab batch and BIOCOIL photobioreactors: experiments and modeling. Bioresource Technology, 211, 327–338.
de Bashan, L. E., & Bashan, Y. (2010). Immobilized microalgae for removing pollutans: review of practical aspects. Bioresource Technology, 101, 1611–1627.
De Francisci, D., Su, Y., Iital, A., & Angelidaki, I. (2018). Evaluation of microalgae production coupled with wastewater treatment. Environmental Technology., 39(5), 581–592.
Estrada, J. M., & Bhamidimarri, R. (2016). A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel, 182, 292–303.
Fernandez, E., Sanchez, E., Bonilla, I., Mateo, P., & Ortega, P. (1984). Effect of boron on the growth and cell composition of Chlorella pyrenoidosa. Phyton, 44, 125–131.
Ferrer, I., & Thurman, E. M. (2015). Chemical constituents and analytical approaches for hydraulic fracturing waters. Trends in Environmental Analytical Chemistry, 5, 18–25.
Frac Focus Chemical Disclosure Registry. (2018). https://fracfocusdata.org/DisclosureSearch/Search.aspx. Accessed on 17 May 2018.
Gallegos, T. J., Varela, B. A., Haines, S. S., & Engle, M. A. (2015). Hydraulic fracturing water use variability in the United States and potential environmental implications. Water Resources. Research., 51(7), 5839–5845.
García, R., Pizarro, C., Lavín, A. G., & Bueno, J. L. (2013). Biomass proximate analysis using thermogravimetry. Bioresource Technology, 139, 1–4.
Godfrey, V. (2012). Production of biodiesel from oleaginous organisms using underutilized wastewaters (MS). Logan, UT: Utah State University.
Haugstad, B. H. (2017). Fuel characterization and process analysis of hydrothermal liquefacion of algae. Grimstad: (MS), University of Agder.
Henley, W. J., Major, K. M., & Hironaka, J. L. (2002). Response to salinity and heat stress in two halotolerant chlorophyte algae. Journal of Phycology, 38(4), 757–766.
Henley, W. J., Hironaka, J. L., Guillou, L., Buchheim, M. A., Buchheim, J. A., Fawley, M. W., et al. (2004). Phylogenetic analysis of the 'Nanochloris-like' algae and diagnoses of Picochlorum oklahomensis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta). Phycologia., 43(6), 641–652.
Hu, M., Chen, Z., Guo, D., Liu, C., Xiao, B., Hu, Z., et al. (2015). Thermogravimetric study on pyrolysis kinetics of Chlorella pyrenoidosa and bloom-forming cyanobacteria. Bioresource Technology, 177, 41–50.
Jackson, R. B., Vengosh, A., Carey, J. W., Davies, R. J., Darrah, T. H., O’Sullivan, F., et al. (2014). The environmental costs and benefits of fracking. Annual Review of Environment and Resources, 39, 327–362.
Kirkwood, A. E., & Henley, W. J. (2006). Algal community dynamics and halotolerance in a terrestrial, hypersaline environment. Journal of Phycology, 42(3), 537–547.
Kirkwood, A. E., Buchheim, J. A., Buchheim, M. A., & Henley, W. J. (2008). Cyanobacterial diversity and halotolerance in a variable hypersaline environment. Microbial Ecology, 55(3), 453–465.
Kirtania, K., & Bhattacharya, S. (2013). Pyrolysis kinetics and reactivity of algae–coal blends. Biomass and Bioenergy, 55, 291–298.
Kondash, A., & Vengosh, A. (2015). Water footprint of hydraulic fracturing. Environmental Sciences and Technology Letters., 2(10), 276–280.
Lammers, P. J., Huesemann, M., Boeing, W., Anderson, D. B., Arnold, R. G., Bai, X., et al. (2017). Review of the cultivation program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Research, 22, 166–186.
Lester, Y., Yacob, T., Morrissey, I., & Linden, K. G. (2014). Can we treat hydraulic fracturing flowback with a conventional biological process? The case of guar gum. Environmental Sciences and Technology Letters., 1(1), 133–136.
Lewin, J. C. (1966). Boron as a growth requirement for diatoms. Journal of Phycology, 2(4), 160–163.
Li, D., Chen, L., Zhao, J., Zhang, X., Wang, Q., Wang, H., et al. (2010). Evaluation of the pyrolytic and kinetic characteristics of Enteromorpha prolifera as a source of renewable bio-fuel from the Yellow Sea of China. Chemical Engineering Research and Design, 88(5), 647–652.
Li, R., Yang, J., Pan, J., Zhang, L., & Qin, W. (2018). Effect of immobilization on growth and organics removal of Chlorella in fracturing flowback fluids treatment. Journal of Environmental Management, 226, 163–168.
Li, R., Pan, J., Yan, M., Yang, J., Qin, W., & Liu, Y. (2020). Treatment of fracturing wastewater using microalgae-bacteria consortium. The Canadian Journal of Chemical Engineering, 98(2), 484–490.
López-González, D., Fernandez-Lopez, M., Valverde, J. L., & Sanchez-Silva, L. (2014). Kinetic analysis and thermal characterization of the microalgae combustion process by thermal analysis coupled to mass spectrometry. Applied Energy, 114, 227–237.
Lutzu, G. A., & Dunford, N. T. (2019a). Algal treatment of wastewater generated during oil and gas production using hydraulic fracturing technology. Environmental Technology., 40(8), 1027–1034.
Lutzu, G. A., & Dunford, N. T. (2019b). Growing algae in produced water generated during oil and gas production using hydraulic fracturing technique. Chemical Engineering Transactions, 74, 1261–1266.
Lutzu, G. A., Locci, A. M., & Cao, G. (2012). Effect of medium composition on the growth of Nannochloris eucaryotum in batch photobioreactors. Journal of Biobased Material and Bioenergy, 6(1), 94–100.
Ma, X., Zhou, W., Fu, Z., Cheng, Y., Min, M., Liu, Y., Zhang, Y., Chen, P., & Ruan, R. (2014). Effect of wastewater-borne bacteria on algal growth and nutrients removal in wastewater-based algae cultivation system. Bioresource Technology, 167, 8–13.
Maddi, B., Viamajala, S., & Varanasi, S. (2011). Comparative study of pyrolysis of algal biomass from natural lake blooms with lignocellulosic biomass. Bioresource Technology, 102(23), 11018–11026.
Maguire-Boyle, S. J., & Barron, A. R. (2014). Organic compounds in produced waters from shale gas wells. Environmental Science: Processes and Impacts, 16(10), 2237–2248.
Mateo, P., Bonilla, I., Fernandez-Valiente, E., & Sanchez-Maeso, E. (1986). Essentiality of boron for dinitrogen fixation in Anabaena sp. PCC 711. Plant Physiology, 81(2), 430–433.
Mauter, M. S., Alvarez, P. J. J., Burton, A., Cafaro, D. C., Chen, W., Gregory, K. B., et al. (2014). Regional variation in water-related impacts of shale gas development and implications for emerging international play. Environmental Science and Technology., 48(15), 8298–8306.
Mayers, J. J., Vaiciulyte, S., Malmhäll-Bah, E., Alcaide-Sancho, J., Ewald, S., Godhe, A., et al. (2018). Identifying a marine microalgae with high carbohydrate productivities under stress and potential for efficient flocculation. Algal Research, 31, 430–442.
Mendes, L.B., Cunha, P. C. R., Montes D’Oca, M., Abreu, P. C., Primel, E. (2010). US Patent 7955505 B2.
NAABB, The National Alliance for Advanced Biofuels and Bioproducts final report. (2014). https://www.energy.gov/sites/prod/files/2014/06/f16/naabb_synopsis_report.pdf. Accessed on 23 October 2014.
NCMA, The National Center for Marine Algae and Microbiota at Boothbay, Maine (NCMA). (2016). https://ncma.bigelow.org/products/algae. Accessed on 23 July 2016.
Notte, C., Allen, D. M., Gehman, J., Alessi, D. S., & Goss, G. G. (2017). Comparative analysis of hydraulic fracturing wastewater practices in unconventional shale developments: regulatory regimes. Canadian. Water Resources. Journal., 42(2), 122–137.
Orosz, M. S., & Forney, D. (2008). A comparison of algae to biofuel conversion pathways for energy storage off-grid. Boston, MA: Massachusetss Institute of Technology.
Racharaks, R., Ge, X., & Li, Y. (2015). Cultivation of marine microalgae using shale gas flowback water and anaerobic digestion effluent as the cultivation medium. Bioresource Technology, 191, 146–156.
Ross, A., Jones, J., Kubacki, M., & Bridgeman, T. (2008). Classification of macroalgae as fuel and its thermochemical behaviour. Bioresource Technology, 99(14), 6494–6504.
Sheehan, J., Dunahay, T., Benemann, J., Roessler, P. (1998). A look back at the US Department of Energy's aquatic species program: biodiesel from algae (Vol. 328): National Renewable Energy Laboratory Golden.
Shores, A., Laituri, M., & Butters, G. (2017). Produced water surface spills and the risk for BTEX and nafthalene groundwater contamination. Water Air and Soil Pollution, 228(11), 435.
Smyth, D. A., & Dugger, W. M. (1981). Cellular changes during boron-deficient culture of the diatom Cylindrotheca fusiformis. Physiologia Plantarum, 51, 111–117.
Soria-Verdugo, A., Goos, E., Morato-Godino, A., García-Hernando, N., & Riedel, U. (2017). Pyrolysis of biofuels of the future: sewage sludge and microalgae – thermogravimetric analysis and modelling of the pyrolysis under different temperature conditions. Energy Conversion and Management, 138, 261–272.
Soru, S., Malavasi, V., Concas, A., Caboni, P., & Cao, G. (2019). A novel investigation of the growth and lipid production of the extremophile microalga Coccomyxa melkonianii SCCA 048 under the effect of different cultivation conditions: Experiments and modeling. Chemical Engineering Journal, 377, 120589.
Stemkovski, M., Baraldi, R., Flores, K. B., & Banks, H. T. (2016). Validation of a mathematical model for green algae (Raphidocelis subcapitata) growth and implications for a coupled dynamical system with Daphnia magna. Applied Sciences, 6(5), 155.
Sudhakar, K., & Premalatha, M. (2015). Characterization of micro algal biomass through FTIR/TGA/CHN analysis: application to Scenedesmus sp. Energy Source. Part A: Recovery, Utilization, and Environmental Effects, 37(21), 2330–2337.
Takáčová, A., Smolinská, M., Ryba, J., Mackuľak, T., Jokrllová, J., Hronec, P., et al. (2014). Biodegradation of Benzo[a]Pyrene through the use of algae. Central European Journal of Chemistry, 12, 1133–1143.
US Environmental Protection Agency (EPA). 1980. Standard method 5220 D. Washington D.C., USA.
UTEX, The culture collection of algae at the University of Texas at Austin (UTEX). (2012). http://web.biosci.utexas.edu/utex/media.aspx. Accessed on 04 December 2012.
Wang, D., Sun, Y., Tsang, D. C., Hou, D., Khan, E., Alessi, D. S., Zhao, Y., Gong, J., & Wang, L. (2020a). The roles of suspended solids in persulfate/Fe2+ treatment of hydraulic fracturing wastewater: Synergistic interplay of inherent wastewater components. Chemical Engineering Journal, 388, 124243.
Wang, D., Sun, Y., Tsang, D. C., Khan, E., Cho, D. W., Zhou, Y., Qi, F., Gong, J., & Wang, L. (2020b). Synergistic utilization of inherent halides and alcohols in hydraulic fracturing wastewater for radical-based treatment: A case study of di-(2-ethylhexyl) phthalate removal. Journal of Hazardous Materials, 384, 121321.
Wood, J. L., Miller, C. D., Sims, R. C., & Takemoto, J. Y. (2015). Biomass and phycocyanin production from cyanobacteria dominated biofilm reactors cultured using oilfield and natural gas extraction produced water. Algal Research, 11, 165–168.
Xu, Y., & Boeing, W. J. (2014). Modeling maximum lipid productivity of microalgae: review and next step. Renewable and Susteneable Energy Reviews, 32, 29–39.
Zhou, N., & Dunford, N. T. (2017a). Characterization of green microalgae and cyanobacteria isolated from Great Salt Plains. Transaction of the ASABE, 60(2), 283–290.
Zhou, N., & Dunford, N. T. (2017b). Thermal degradation profile and microwave aided pyrolysis of green algae and cyanobacteria isolated from Great Salt Plains. Transaction of the ASABE, 60, 561–569.
Zhu, Y., & Dunford, N. T. (2013). Growth and biomass characteristics of Picochlorum oklahomensis and Nannochloropsis oculata. Journal of the American Oil Chemists' Society, 90(6), 841–849.
Acknowledgements
This research was supported by the Oklahoma Center for the Advancement of Science and Technology, Basic Plant Science Program, Project # PS13-007, and Oklahoma Water Resources Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lutzu, G.A., Marin, M.A., Concas, A. et al. Growing Picochlorum oklahomensis in Hydraulic Fracturing Wastewater Supplemented with Animal Wastewater. Water Air Soil Pollut 231, 457 (2020). https://doi.org/10.1007/s11270-020-04826-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04826-1