Abstract
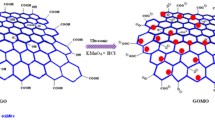
In this study, graphene oxide (GO) was prepared using modified Hummer’s method and doped with Fe-Ni nanoparticles. Morphological characterization of the Fe-Ni nanoparticles showed flake-like structure correlating to taenite phase, while Raman spectroscopy suggested that graphene oxide was multi-layered. Batch adsorption experiments were performed to determine the effect of solution pH, initial uranium (VI) concentration, adsorbent dosage, contact time, and specific anions (SO42− and NO32−) on the adsorption of U (VI). Solution pH had significant effect on U (VI) sorption on Fe-Ni/GO, with maximum removal of 98.4% at pH 4, while it was 98% at pH 8 for GO. Sorption kinetics revealed fast adsorption within 15 min. The kinetic and equilibrium data was evaluated using pseudo-first-order, pseudo-second-order, Elovich, Langmuir, Freundlich, and Temkin models. The mechanism of U (VI) sorption appeared to be a combination of chemisorption and possible pore diffusion of the U (VI) moieties to the porous structure of GO and Fe-Ni/GO. Overall, Fe-Ni/GO was a better adsorbent than GO with higher sorption capacities. U (VI) sorption on GO and Fe-Ni/GO followed pseudo-second-order reaction kinetics (R2 > 0.99). Good fit to Langmuir isotherm model (R2 > 0.98) suggested favorable monolayer adsorption, with a maximum U (VI) adsorption capacity on Fe-Ni/GO to be 25.64 mg/g. Moderate to insignificant effect of specific anions even at high concentrations on U (VI) removal capacities makes Fe-Ni/GO an excellent candidate.










Similar content being viewed by others
References
Agrawal, Y. K., Shrivastav, P., & Menon, S. K. (2000). Solvent extraction, separation of uranium (VI) with crown ether. Separation and Purification Technology, 20(2–3), 177–183. https://doi.org/10.1016/S1383-5866(00)00110-6.
Amphlett, J. T. M., Choi, S., Parry, S. A., Moon, E. M., Sharrad, C. A., & Ogden, M. D. (2019). Insights on uranium uptake mechanisms by ion exchange resins with chelating functionalities: chelation vs. anion exchange. Chemical Engineering Journal, 123712. https://doi.org/10.1016/j.cej.2019.123712.
Bhalara, P. D., Punetha, D., & Balasubramanian, K. (2014). A review of potential remediation techniques for uranium(VI) ion retrieval from contaminated aqueous environment. Journal of Environmental Chemical Engineering, 2(3), 1621–1634. https://doi.org/10.1016/j.jece.2014.06.007.
Chen, J., Yao, B., Li, C., & Shi, G. (2013). An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon, 64(1), 225–229. https://doi.org/10.1016/j.carbon.2013.07.055.
Coyte, R. M., Jain, R. C., Srivastava, S. K., Sharma, K. C., Khalil, A., Ma, L., & Vengosh, A. (2018). Large-scale uranium contamination of groundwater resources in India. Environmental Science and Technology Letters, 5(6), 341–347. https://doi.org/10.1021/acs.estlett.8b00215.
Dong, Q., Qu, W., Liang, W., Tai, F., Guo, K., Leung, C.-W., et al. (2016). Porphyrin-based metallopolymers: synthesis, characterization and pyrolytic study for the generation of magnetic metal nanoparticles. Journal of Materials Chemistry C, 4(22), 5010–5018. https://doi.org/10.1039/C6TC00145A.
Dreyer, D. R., Park, S., Bielawski, C. W., & Ruoff, R. S. (2010). The chemistry of graphene oxide. Chemical Society Reviews, 39(1), 228–240. https://doi.org/10.1039/b917103g.
Duff, M. C., Coughlin, J. U., & Hunter, D. B. (2002). Uranium co-precipitation with iron oxide minerals. Geochimica et Cosmochimica Acta, 66(20), 3533–3547. https://doi.org/10.1016/S0016-7037(02)00953-5.
Elabd, A. A., Zidan, W. I., Abo-Aly, M. M., Bakier, E., & Attia, M. S. (2014). Uranyl ions adsorption by novel metal hydroxides loaded Amberlite IR120. Journal of Environmental Radioactivity, 134, 99–108. https://doi.org/10.1016/j.jenvrad.2014.02.008.
Fan, F.-L., Qin, Z., Bai, J., Rong, W.-D., Fan, F.-Y., Tian, W., et al. (2012). Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@SiO2 composite particles. Journal of Environmental Radioactivity, 106, 40–46. https://doi.org/10.1016/j.jenvrad.2011.11.003.
Gu, B., Ku, Y. K., & Jardine, P. M. (2004). Sorption and binary exchange of nitrate, sulfate, and uranium on an anion-exchange resin. Environmental Science and Technology, 38(11), 3184–3188. https://doi.org/10.1021/es034902m.
Guo, S., Wang, L., & Wu, H. (2015). Facile synthesis and enhanced electromagnetic wave absorption of thorny-like Fe–Ni alloy/ordered mesoporous carbon composite. Advanced Powder Technology, 26(4), 1250–1255. https://doi.org/10.1016/j.apt.2015.06.007.
Han, R., Zou, W., Wang, Y., & Zhu, L. (2007). Removal of uranium(VI) from aqueous solutions by manganese oxide coated zeolite: discussion of adsorption isotherms and pH effect. Journal of Environmental Radioactivity, 93(3), 127–143. https://doi.org/10.1016/j.jenvrad.2006.12.003.
Huang, X., Yin, Z., Wu, S., Qi, X., He, Q., Zhang, Q., et al. (2011). Graphene-based materials: synthesis, characterization, properties, and applications. Small, 7(14), 1876–1902. https://doi.org/10.1002/smll.201002009.
Jakhu, R., Mehra, R., & Mittal, H. M. (2016). Exposure assessment of natural uranium from drinking water. Environmental Science: Processes and Impacts, 18, 1540–1549. https://doi.org/10.1039/c6em00514d.
Jegadeesan, G. B., & Lalvani, S. B. (2017). Selenium reduction on Ni-Fe bimetallic nanoparticles: effect of process variables on reaction rates. Desalination and Water Treatment, 67(20449), 292–299. https://doi.org/10.5004/dwt.2017.20449.
Katz, S. A. (2014). The chemistry and toxicology of depleted uranium. Toxics, 2(1), 50–78. https://doi.org/10.3390/toxics2010050.
Kumar, A. S. K., Kakan, S. S., & Rajesh, N. (2013). A novel amine impregnated graphene oxide adsorbent for the removal of hexavalent chromium. Chemical Engineering Journal, 230, 328–337. https://doi.org/10.1016/j.cej.2013.06.089.
Li, J., & Zhang, Y. (2012). Remediation technology for the uranium contaminated environment: a review. Procedia Environmental Sciences, 13, 1609–1615. https://doi.org/10.1016/j.proenv.2012.01.153.
Li, Z., Chen, F., Yuan, L., Liu, Y., Zhao, Y., Chai, Z., & Shi, W. (2012). Uranium(VI) adsorption on graphene oxide nanosheets from aqueous solutions. Chemical Engineering Journal, 210, 539–546. https://doi.org/10.1016/j.cej.2012.09.030.
Li, Z. J., Wang, L., Yuan, L. Y., Xiao, C. L., Mei, L., Zheng, L. R., et al. (2015a). Efficient removal of uranium from aqueous solution by zero-valent iron nanoparticle and its graphene composite. Journal of Hazardous Materials, 290, 26–33. https://doi.org/10.1016/j.jhazmat.2015.02.028.
Li, L., Xu, M., Chubik, M., Chubik, M., Gromov, A., Wei, G., & Han, W. (2015b). Entrapment of radioactive uranium from wastewater by using fungus-Fe3O4 bio-nanocomposites. RSC Advances, 5(52), 41611–41616. https://doi.org/10.1039/c5ra03643g.
Li, M., Liu, H., Chen, T., Dong, C., & Sun, Y. (2019). Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption. Science of the Total Environment, 651, 1020–1028. https://doi.org/10.1016/j.scitotenv.2018.09.259.
Lian, P., Zhu, X., Liang, S., Li, Z., Yang, W., & Wang, H. (2010). Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries. Electrochimica Acta, 55(12), 3909–3914. https://doi.org/10.1016/j.electacta.2010.02.025.
Lovley, D. R., & Phillips, E. J. P. (1992). Bioremediation of uranium contamination with enzymatic uranium reduction. Environmental Science and Technology, 26(11), 2228–2234. https://doi.org/10.1021/es00035a023.
Luo, X., Wang, C., Luo, S., Dong, R., Tu, X., & Zeng, G. (2012). Adsorption of As (III) and As (V) from water using magnetite Fe 3O 4-reduced graphite oxide-MnO 2 nanocomposites. Chemical Engineering Journal, 187, 45–52. https://doi.org/10.1016/j.cej.2012.01.073.
Massey, M. S., Lezama-Pacheco, J. S., Jones, M. E., Ilton, E. S., Cerrato, J. M., Bargar, J. R., & Fendorf, S. (2014). Competing retention pathways of uranium upon reaction with Fe(II). Geochimica et Cosmochimica Acta, 142, 166–185. https://doi.org/10.1016/j.gca.2014.07.016.
Nilchi, A., Shariati Dehaghan, T., & Rasouli Garmarodi, S. (2013). Kinetics, isotherm and thermodynamics for uranium and thorium ions adsorption from aqueous solutions by crystalline tin oxide nanoparticles. Desalination, 321, 67–71. https://doi.org/10.1016/j.desal.2012.06.022.
O’Loughlin, E. J., Kelly, S. D., Cook, R. E., Csencsits, R., & Kemner, K. M. (2003). Reduction of uranium(VI) by mixed iron(II)/iron(III) hydroxide (green rust): formation of UO2 nanoparticles. Environmental Science and Technology, 37(4), 721–727. https://doi.org/10.1021/es0208409.
Pant, D., Keesari, T., Roy, A., Sinha, U. K., Singh, M., Jain, S. K., & Tripathi, R. M. (2019). Study on groundwater quality in parts of Rajasthan with special reference to uranium contamination. Journal of Radioanalytical and Nuclear Chemistry, 322, 165–171. https://doi.org/10.1007/s10967-019-06525-6.
Quesada-González, D., Jairo, G. A., Blake, R. C., Blake, D. A., & Merkoçi, A. (2018). Uranium (VI) detection in groundwater using a gold nanoparticle/paper-based lateral flow device. Scientific Reports, 8(1), 16157. https://doi.org/10.1038/s41598-018-34610-5.
Roberts, H. E., Morris, K., Law, G. T. W., Mosselmans, J. F. W., Bots, P., Kvashnina, K., & Shaw, S. (2017). Uranium(V) incorporation mechanisms and stability in Fe(II)/Fe(III) (oxyhydr)oxides. Environmental Science and Technology Letters, 4(10), 421–426. https://doi.org/10.1021/acs.estlett.7b00348.
Sadeghi, S., Azhdari, H., Arabi, H., & Moghaddam, A. Z. (2012). Surface modified magnetic Fe3O4 nanoparticles as a selective sorbent for solid phase extraction of uranyl ions from water samples. Journal of Hazardous Materials, 215–216, 208–216. https://doi.org/10.1016/j.jhazmat.2012.02.054.
Selvakumar, A., & Rangabhashiyam, S. (2019). Biosorption of Rhodamine B onto novel biosorbents from Kappaphycus alvarezii, Gracilaria salicornia and Gracilaria edulis. Environmental Pollution, 255, 113291. https://doi.org/10.1016/j.envpol.2019.113291.
Sepehrian, H., Samadfam, M., & Asadi, Z. (2012). Studies on the recovery of uranium from nuclear industrial effluent using nanoporous silica adsorbent. International journal of Environmental Science and Technology, 9(4), 629–636. https://doi.org/10.1007/s13762-012-0065-3.
Shao, L., Wang, X., Ren, Y., Wang, S., Zhong, J., Chu, M., et al. (2016). Facile fabrication of magnetic cucurbit[6]uril/graphene oxide composite and application for uranium removal. Chemical Engineering Journal, 286, 311–319. https://doi.org/10.1016/j.cej.2015.10.062.
Shen, J., & Schäfer, A. (2014). Removal of fluoride and uranium by nanofiltration and reverse osmosis: a review. Chemosphere, 117, 679–691. https://doi.org/10.1016/j.chemosphere.2014.09.090.
Shukla, J. P., Kumar, A., & Singh, R. K. (1993). Solvent extraction of uranium(VI) into toluene by dicyclohexano-18-crown-6 from mixed aqueous-organic solutions. Talanta, 40(8), 1261–1266. https://doi.org/10.1016/0039-9140(93)80196-X.
Subbiah, D. K., Babu, K. J., Das, A., & Rayappan, J. B. B. (2019). NiOx Nanoflower modified cotton fabric for UV filter and gas sensing applications. ACS Applied Materials & Interfaces, 11(22), 20045–20055. https://doi.org/10.1021/acsami.9b04682.
Sun, Y., Ding, C., Cheng, W., & Wang, X. (2014). Simultaneous adsorption and reduction of U (VI) on reduced graphene oxide-supported nanoscale zerovalent iron. Journal of Hazardous Materials, 280, 399–408. https://doi.org/10.1016/j.jhazmat.2014.08.023.
Tan, L., Liu, Q., Jing, X., Liu, J., Song, D., Hu, S., et al. (2015). Removal of uranium(VI) ions from aqueous solution by magnetic cobalt ferrite/multiwalled carbon nanotubes composites. Chemical Engineering Journal, 273, 307–315. https://doi.org/10.1016/j.cej.2015.01.110.
Thiagarajan, R., Swarup, R., & Patil, S. K. (1979). Separation of uranium and plutonium from an aqueous solution containing phosphoric, sulfuric, and nitric acids by solvent extraction. Separation Science and Technology, 14(8), 749–755. https://doi.org/10.1080/01496397908060235.
Wazne, M., Korfiatis, G. P., & Meng, X. (2003). Carbonate effects on hexavalent uranium adsorption by iron oxyhydroxide. Environmental Science and Technology, 37(16), 3619–3624. https://doi.org/10.1021/es034166m.
Wu, F.-C., Tseng, R.-L., & Juang, R.-S. (2009). Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chemical Engineering Journal, 153(1), 1–8. https://doi.org/10.1016/j.cej.2009.04.042.
Xie, Y., Chen, C., Ren, X., Wang, X., Wang, H., & Wang, X. (2019). Emerging natural and tailored materials for uranium-contaminated water treatment and environmental remediation. Progress in Materials Science, 103, 180–234. https://doi.org/10.1016/j.pmatsci.2019.01.005.
Yadav, S. K., Ramanathan, A. L., Kumar, M., Chidambaram, S., Gautam, Y. P., & Tiwari, C. (2020). Assessment of arsenic and uranium co-occurrences in groundwater of central Gangetic Plain, Uttar Pradesh, India. Environmental Earth Sciences, 79(6), 1–14. https://doi.org/10.1007/s12665-020-8892-x.
Yekta, S., Sadeghi, M., & Ghaedi, H. (2016). Removal of uranium (U(VI)) ions using NiO NPs/Ag-clinoptilolite zeolite composite adsorbent from drinking water: equilibrium, kinetic and thermodynamic studies. Int. J. Bio-Inorg. Hybr. Nanomater, 5(4), 279–295.
Yu, S., Yin, L., Pang, H., Wu, Y., Wang, X., Zhang, P., et al. (2018). Constructing sphere-like cobalt-molybdenum-nickel ternary hydroxide and calcined ternary oxide nanocomposites for efficient removal of U(VI) from aqueous solutions. Chemical Engineering Journal, 352, 360–370. https://doi.org/10.1016/j.cej.2018.07.033.
Zhang, J., Guo, Z., Li, Y., Pan, S., Chen, X., & Xu, J. (2016). Effect of environmental conditions on the sorption of uranium on Fe3O4@MnO2 hollow spheres. Journal of Molecular Liquids, 223, 534–540. https://doi.org/10.1016/j.molliq.2016.07.136.
Zhang, Q., Zhao, D., Ding, Y., Chen, Y., Li, F., Alsaedi, A., et al. (2019). Synthesis of Fe–Ni/graphene oxide composite and its highly efficient removal of uranium(VI) from aqueous solution. Journal of Cleaner Production, 230, 1305–1315. https://doi.org/10.1016/j.jclepro.2019.05.193.
Zong, P., Wang, S., Zhao, Y., Wang, H., Pan, H., & He, C. (2013). Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(VI) from aqueous solutions. Chemical Engineering Journal, 220, 45–52. https://doi.org/10.1016/j.cej.2013.01.038.
Acknowledgments
The authors acknowledge the support of SASTRA Deemed University for their help and support in completion of the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1520 kb)
Rights and permissions
About this article
Cite this article
Rohith, S., Ramanan, K.K., Srinivas, N.S. et al. Fe-Ni-Doped Graphene Oxide for Uranium Removal—Kinetics and Equilibrium Studies. Water Air Soil Pollut 231, 444 (2020). https://doi.org/10.1007/s11270-020-04804-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04804-7