Abstract
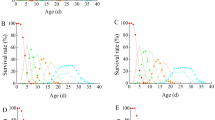
We investigated (1) whether rapid acquisition of tolerance to pirimicarb would develop in three cladocerans (Daphnia magna, Scapholeberis kingi, and Ceriodaphnia cornuta) after short-term exposure and whether this tolerance was maintained in their descendants over four generations; (2) whether tolerance implies fitness costs, and whether these costs quantitatively correlated with tolerance levels; and (3) how AChE activity and AChE mRNA levels were altered by short-term exposure to pirimicarb. After 48 h of exposure to 0, 1.3, 2.5, 5.0, 10.0, 20.0, and 40.0 μg/L pirimicarb only in F0 generation, the surviving cladocerans in the concentrations (0, 1.3, 2.5, 5.0, 10.0 μg/L) were subsequently kept for additional three generations (F1, F2, and F3) in the absence of pirimicarb. Among the three tested cladocerans, the EC50 value (50% effective concentration for 48 h exposure, using immobility as the endpoint) of only C. cornuta increased significantly. The increased tolerance in C. cornuta was retained in F1, F2, and F3. In C. cornuta, AChE activity and AChE mRNA levels in F0 decreased significantly, but these values in F1 were comparable to those in the controls, suggesting these changes may be related to the tolerance. Direct exposure of F0 C. cornuta to 2.5 μg/L or 5.0 μg/L pirimicarb induced a decrease in intrinsic population growth rate. However, this effect disappeared as soon as exposure was removed in F1. Thus, tolerance to pirimicarb, as observed in C. cornuta, involves no fitness cost. Our findings will contribute to clarifying adaptation of aquatic organisms to chemicals.






Similar content being viewed by others
References
Agra, A. R., Guilhermino, L., Soares, A. M. V. M., & Barata, C. (2010). Genetic costs of tolerance to metals in Daphnia longispina populations historically exposed to a copper mine drainage. Environmental Toxicology and Chemistry, 29(4), 939–946.
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19, 716–723.
Amarasinghe, P. B., Boersma, M., & Vijverberg, J. (1997). The effect of temperature, and food quantity and quality on the growth and development rates in laboratory-cultured copepods and cladocerans from a Sri Lankan reservoir. Hydrobiologia, 350, 131–144.
Andersen, T. H., Tjørnhøj, R., Wollenberger, Slothuus, T., & Baun, A. (2006). Acute and chronic effects of pulse exposure of Daphnia magna to dimethoate and pirimicarb. Environmental Toxicology and Chemistry, 25(5), 1187–1195.
Antunes, S. C., Castro, B. B., & Gonçalves, F. (2004). Effect of food level on the acute and chronic responses of daphnids to lindane. Environmental Pollution, 127, 367–375.
Asselman, J., De Coninck, D. I. M., Vandegehuchte, M. B., Beert, E., Janssen, C. R., Orsini, L., et al. (2017). Bisulfite sequencing with Daphnia highlights a role for epigenetics in regulating stress response to Microcystis through preferential differential methylation of serine and threonine amino acids. Environmental Science & Technology, 51(2), 924–931.
Bae, E., Samanta, P., Yoo, J., & Jung, J. (2016). Effects of multigenerational exposure to elevated temperature on reproduction, oxidative stress, and Cu toxicity in Daphnia magna. Ecotoxicology and Environmental Safety, 132, 366–371.
Baird, D. J., Barber, I., Bradley, M. C., Soares, A. M. V. M., & Calow, P. (1991). A comparative study of genotype sensitivity to acute toxic stress using clones of Daphnia magna Straus. Ecotoxicology and Environmental Safety, 21, 257–265.
Barata, C., & Baird, D. J. (1998). Phenotypic plasticity and constancy of life-history traits in laboratory clones of Daphnia magna Straus: effects of neonatal length. Functional Ecology, 12, 442–452.
Barata, C., Baird, D. J., Soares, A. M. V. M., & Guilhermino, L. (2001). Biochemical factors contributing to response variation among resistant and sensitive clones of Daphnia magna Straus exposed to ethyl parathion. Ecotoxicology and Environmental Safety, 49, 155–163.
Barbosa, C., Lopes, I., Venâncio, C., Silva, J., Janeiro, M. J., Morrissey, M. B., & Soares, A. M. V. M. (2015). Maternal response to environmental unpredictability. Ecology and Evolution, 5(20), 4567–4577.
Benting, J., & Nauen, R. (2004). Biochemical evidence that an S431F mutation in acetylcholinesterase-1 of Aphis gossypii mediates resistance to pirimicarb and omethoate. Pest Management Science, 60(11), 1051–1055.
Boxall, A. B., Fogg, L. A., Ashauer, R., Bowles, T., Sinclair, C. J., Colyer, A., et al. (2013). Effects of repeated pulsed herbicide exposures on the growth of aquatic macrophytes. Environmental Toxicology and Chemistry, 32(1), 193–200.
Brausch, J. M., & Smith, P. N. (2009). Development of resistance to cyfluthrin and naphthalene among Daphnia magna. Ecotoxicology, 18(5), 600–609.
Brock, T. C. M., & Wijngaarden, R. P. A. V. (2012). Acute toxicity tests with Daphnia magna, Americamysis bahia, Chironomus riparius and Gammarus pulex and implications of new EU requirements for the aquatic effect assessment of insecticides. Environmental Science and Pollution Research, 19(8), 3610–3618.
Carroll, S. P., Jørgensen, P. S., Kinnison, M. T., Bergstrom, C. T., Denison, R. F., Gluckman, P., et al. (2014). Applying evolutionary biology to address global challenges. Science, 346(6207).
Case, T. J. (2000). An illustrated guide to theoretical ecology. New York: Oxford University.
Charpentier, A., & Fournier, D. (2001). Levels of total acetylcholinesterase in Drosophila melanogaster in relation to insecticide resistance. Pesticide Biochemistry and Physiology, 70, 100–107.
Coustau, C., Chevillon, C., & Ffrench-Constant, R. (2000). Resistance to xenobiotics and parasites: can we count the cost? Trends in Ecology & Evolution, 15(9), 378–383.
Débarre, F. (2015). Fitness costs in spatially structured environments. Evolution, 69-65(5), 1329–1335.
Deng, N., Allison, J. J., Fang, H. J., Ash, A. S., & Ware Jr., J. E. (2013). Using the bootstrap to establish statistical significance for relative validity comparisons among patient-reported outcome measures. Health and Quality of Life Outcomes, 11, 89.
Ebrahimpour, M., Alipour, H., & Rakhshah, S. (2010). Influence of water hardness on acute toxicity of copper and zinc on fish. Toxicology and Industrial Health, 26(6), 361–365.
Efron, B., & Tibshirani, R. (1993). An introduction to the bootstrap. . (pp. 52) New York: Chapman and Hall.
Elendt, B. P., & Bias, W.-R. (1990). Trace nutrient deficiency in Daphnia magna cultured in standard medium for toxicity testing. Effects of the optimization of culture conditions on life history parameters of D. magna. Water Research, 24(9), 1157–1167.
Enserink, L., Luttmer, W., & Maas-Diepeveen, H. (1990). Reproductive strategy of Daphnia magna affects the sensitivity of its progeny in acute toxicity tests. Aquatic Toxicology, 17, 15–25.
Forbes, V. E., & Depledge, M. H. (1992). Predicting population response to pollutants: the significance of sex. Functional Ecology, 6(4), 376–381.
Gerhard, D., & Ritz, C. (2017). Marginalization in nonlinear mixed-effects models with an application to dose-response analysis. https://arxiv.org/abs/1707.02502. Accessed 6 Jan 2020.
Gliwicz, Z. M., & Guisande, C. (1992). Family planning in Daphnia: resistance to starvation in offspring born to mothers grown at different food levels. Oecologia, 91, 463–467.
Haap, T., & Köhler, H.-R. (2009). Cadmium tolerance in seven Daphnia magna clones is associated with reduced hsp70 baseline levels and induction. Aquatic Toxicology, 94(2), 131–137.
Hayasaka, D., Korenaga, T., Suzuki, K., Sanchez-Bayo, F., & Goka, K. (2012). Differences in susceptibility of five cladoceran species to two systemic insecticides, imidacloprid and fipronil. Ecotoxicology, 21(2), 421–427.
Heine-Fuster, I., Aránguiz-Acuña, A., & Ramos-Jiliberto, R. (2017). Pesticide increases transgenerational cost of inducible defenses in a freshwater rotifer. Hydrobiologia, 799, 249–260.
Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6, 65–70.
Hong, L. C. D., Slooten, K. B. V., & Tarradellas, J. (2004). Tropical ecotoxicity testing with Ceriodaphnia cornuta. Environmental Toxicology, 19(5), 497–504.
Hosmer, D., & Lemeshow, S. (2000). Applied logistic regression. New York: Wiley.
Hua, J., Jones, D. K., & Relyea, R. A. (2014). Induced tolerance from a sublethal insecticide leads to cross-tolerance to other insecticides. Environmental Science & Technology, 48(7), 4078–4085.
Hyne, R. V., Pablo, F., Julli, M., & Markich, S. J. (2005). Influence of water chemistry on the acute toxicity of copper and zinc to the cladoceran Ceriodaphnia cf dubia. Environmental Toxicology and Chemistry, 24(7), 1667–1675.
Ingersoll, C. G., & Winner, R. W. (1982). Effect on Daphnia pulex (De Geer) of daily pulse exposures to copper or cadmium. Environmental Toxicology and Chemistry, 1(4), 321–327.
Ishimota, M., & Tomiyama, N. (2020). Increased acetylcholinesterase inhibitor sensitivity as an intergenerational response to short-term acetylcholinesterase inhibitor exposure in Scapholeberis kingi. Limnology, 21, 187–196. https://doi.org/10.1007/s10201-019-00598-8.
Ishimota, M., Tajiki-Nishino, R., Fukuyama, T., & Tomiyama, N. (2020). Rapid adaptation of Chironomus yoshimatsui to acetylcholinesterase inhibitors (Pyraclofos and Pirimicarb) in a multi-generation study. Journal of Environmental Science and Health, Part B. https://doi.org/10.1080/03601234.2019.1708165.
Jansen, M., Stoks, R., Coors, A., van Doorslaer, W., & De Meester, L. (2011). Collateral damage: rapid exposure-induced evolution of pesticide resistance leads to increased susceptibility to parasites. Evolution, 65(9), 2681–2691.
Jeon, J., Kretschmann, A., Escher, B., & Hollender, J. (2013). Characterization of acetylcholinesterase inhibition and energy allocation in Daphnia magna exposed to carbaryl. Ecotoxicology and Environmental Safety, 98, 28–35.
Jesus, F. T., Martins, C., & Nogueria, J. A. (2014). Changes in life-history parameters of Daphnia longispina (Cladocera, Crustacea) as a function of water chemistry. Journal of Limnology, 73(2), 340–349.
Jiang, X., Liang, H., Yang, W., Zhang, J., Zhao, Y., Chen, L., et al. (2013). Fitness benefits and costs of induced defenses in Daphnia carinata (Cladocera: Daphnidae) exposed to cyanobacteria. Hydrobiologia, 702, 105–113.
Kilham, S. S., Kreeger, D. A., Lynn, S. G., Goulden, C. E., & Herrera, L. (1998). COMBO: a defined freshwater culture medium for algae and zooplankton. Aquatic Toxicology, 17, 15–26.
Knudsen, T. M., Rezwan, F. I., Jiang, Y., Karmaus, W., Svanes, C., & Holloway, J. W. (2018). Transgenerational and intergenerational epigenetic inheritance in allergic diseases. Journal of Allergy and Clinical Immunology, 142(3), 765–772.
Lacy, R. C. (1987). Loss of genetic diversity from managed populations: interacting effects of drift mutation, selection, and population subdivision. Conservation Biology, 1, 143–158.
Laskowski, R. (2001). Why short-term bioassays are not meaningful – effects of a pesticide (imidacloprid) and a metal (cadmium) on pea aphids (Acyrthosiphon pisum Harris). Ecotoxicology, 10, 177–183.
Li, S., Xu, J., & Sheng, L. (2014). The trans-generation effect during pulsed cadmium exposure: tolerance and induction of hsp70. Ecotoxicology and Environmental Safety, 107, 300–305.
Li, S., Sheng, L., Xu, J., Tong, H., & Jiang, H. (2016). The induction of metallothioneins during pulsed cadmium exposure to Daphnia magna: recovery and trans-generational effect. Ecotoxicology and Environmental Safety, 126, 71–77.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods, 25, 402–408.
Lu, Y. H., He, Y. P., & Gao, X. W. (2013). Comparative studies on acetylcholinesterase characteristics between the aphids, Sitobion avenae and Rhopalosiphum padi. Journal of Insect Science, 13, 9.
Mano, H., & Tanaka, Y. (2017). Spatial difference in genetic variation for fenitrothion tolerance between local populations of Daphnia galeata in Lake Kasumigaura, Japan. Ecotoxicology, 26, 1358–1365.
Mano, H., Sakamoto, M., & Tanaka, Y. (2010). A comparative study of insecticide toxicity among seven cladoceran species. Ecotoxicology, 19, 1620–1625.
Marques, S. C., Azeiteiro, U. M., Leandro, S. M., Queiroga, H., Primo, A. L., Martinho, F., et al. (2008). Predicting zooplankton response to environmental changes in a temperate estuarine ecosystem. Marine Biology, 155, 531–541.
McMahon, J. W., & Rigler, F. H. (1965). Feeding rate of Daphnia magna Straus in different foods labelled with radioactive phosphorus. Limnology and Oceanography. https://doi.org/10.4319/lo.1965.10.1.0105.
Medina, M. H., Correa, J. A., & Barata, C. (2007). Micro-evolution due to pollution: possible consequences for ecosystem responses to toxic stress. Chemosphere, 67, 2105–2114.
Menzel, S., Bouchnak, R., Menzel, R., & Steinberg, C. E. W. (2011). Dissolved humic substances initiate DNA-methylation in cladocerans. Aquatic Toxicology, 105(3–4), 640–642.
Mireji, P. O., Keating, J., Hassanali, A., Mbogo, C. M., Muturi, M. N., Githure, J. I., et al. (2010). Biological cost of tolerance to heavy metals in the mosquito Anpheles gambiae. Medical and Veterinary Entomology, 24(2), 101–107.
Mousseau, T. A., Uller, T., Wapstra, E., & Badyaev, A. V. (2009). Evolution of maternal effects: past and present. Philosophical Transactions of the Royal Society B, 364(1520), 1035–1038.
Muthusamy, R., Ramkumar, G., Karthi, S., & Shivakumar, M. S. (2014). Biochemical mechanisms of insecticide resistance in field population of dengue vector Aedes aegypti (Diptera: Culicidae). International Journal of Mosquito Research, 1(2), 1–4.
Nandini, S., & Sarma, S. S. S. (2003). Population growth of some genera of cladocerans (Cladocera) in relation to algal food (Chlorella vulgaris) levels. Hydrobiologia, 491, 211–219.
Nkya, T. E., Akhouayri, I., Kisinza, W., & David, J. P. (2013). Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochemistry and Molecular Biology, 43, 407–416.
OECD (Organization for Economic Co-operation and Development) (1997). OECD guidelines for testing of chemicals: No.211: Daphnia magna reproduction test.
OECD (Organization for Economic Co-operation and Development) (2004). OECD guideline for the testing of chemicals, section 2, test No.202: Daphnia sp., acute immobilization test.
Parveen, S., & Mola, H. R. A. (2013). Comparison of physico-chemical parameters and zooplankton diversity in two perennial ponds at Aligarh, India. Journal of Environmental Biology, 34, 709–716.
Pieters, B. J., & Liess, M. (2006). Maternal nutritional state determines the sensitivity of Daphnia magna offspring to short-term Fenvalerate exposure. Aquatic Toxicology, 76(3–4), 268–277.
R Development Core Team (2019). R: a language and environment for statistical computing. Version 3.6.1. R Foundation for Statistical Computing, Vienna, Austria.
Ribeiro, R., & Lopes, I. (2013). Contaminant driven genetic erosion and associated hypotheses on alleles loss, reduced population growth rate and increased susceptibility to future stressors: an essay. Ecotoxicology, 22, 889–899.
Ritz, C., Cedergreen, N., Jensen, J. E., & Streibig, J. C. (2006). Relative potency in nonsimilar dose–response curves. Weed Science, 54(3), 407–412.
Saro, L., Lopes, I., Martins, N., & Ribeiro, R. (2012). Testing hypotheses on the resistance to metals by Daphnia longispina: differential acclimation, endpoints association, and fitness costs. Environmental Toxicology and Chemistry, 31(4), 909–915.
Snell, T. W., Moffat, B. D., & Janssen, C. (1991). Acute toxicity tests using rotifers. Ecotoxicology and Environmental Safety, 21, 308–317.
Stamatis, N., Hela, D., Triantafyllidis, V., Konstantinou, I., & Konstantinou, I. (2013). Spatiotemporal variation and risk assessment of pesticides in water of the lower Catchment Basin of Acheloos River, Western Greece. The Scientific World Journal, 2013, 231610.
Stehle, S., Knäbel, A., & Schulz, R. (2013). Probabilistic risk assessment of insecticide concentrations in agricultural surface waters: a critical appraisal. Environmental Monitoring and Assessment, 185(8), 6295–6310.
Struger, J., Grabuski, J., Cagampan, S., Sverko, E.d., & Marvin, C. (2016). Occurrence and distribution of carbamate pesticides and metalaxyl in southern Ontario surface waters 2007–2010. Bulletin of Environmental Contamination and Toxicology, 96, 423–431.
Taberner, A., Castañera, P., Silvestre, E., & Dopazo, J. (1993). Estimation of the intrinsic rate of natural increase and its error by both algebraic and resampling approaches. Computer Applications in the Biosciences, 9(5), 535–540.
Terra, N. R., & Feiden, I. R. (2003). Reproduction and survival of Daphnia magna Straus, 1820 (Crustácea: Cladocera) under different hardness condition. Acta Limnologica Brasiliensia, 15(1), 51–55.
Thomas, C. R., Hose, G. C., Warne, S. J. M., & Lim, R. P. (2008). Effects of river water and salinity on the toxicity of Deltamethrin to freshwater shrimp, Cladoceran, and fish. Archives of Environmental Contamination and Toxicology, 55, 610–618.
Toumi, H., Boumaiza, M., Millet, M., Radetski, C. M., Felten, V., & Férard, J. F. (2015). Is acetylcholinesterase a biomarker of susceptibility in Daphnia magna (Crustacea, Cladocera) after deltamethrin exposure? Chemosphere, 120, 351–356.
Trac, L. N., Andersen, O., & Palmqvist, A. (2016). Deciphering mechanisms of malathion toxicity under pulse exposure of the freshwater cladoceran Daphnia magna. Environmental Toxicology and Chemistry, 35(2), 394–404.
Uku, J. N., & Mavuti, K. M. (1994). Comparative limnology, species diversity and biomass relationship of zooplankton and phytoplankton in five freshwater lakes in Kenya. Hydrobiologia, 272, 251–258.
US EPA (United States Environmental Protection Agency Office of Chemical Safety and Pollution Prevention) (2012). Ecological effects guideline, OCSPP 850.6100. Environmental chemistry methods and associated independent laboratory validation. Washington, D.C: U.S. Environmental Protection Agency.
Venâncio, C., Ribeiro, R., Soares, A. M. V. M., & Lopes, I. (2018). Multigenerational effects of salinity in six clonal lineages of Daphnia longispina. Science of the Total Environment, 619–620, 194–202.
Wang, L., Shi, X., Su, Y., Meng, Z., & Lin, H. (2012). Loss of genetic diversity in the cultured stocks of the large yellow croaker, Larimichthys crocea, revealed by microsatellites. International Journal of Molecular Sciences, 13, 5584–5597.
Ward, T., & Robinson, W. E. (2005). Evaluation of cadmium resistance in Daphnia magna. Environmental Toxicology and Chemistry, 24(9), 2341–2349.
Xiao, D., Yang, T., Desneux, N., Han, P., & Gao, X. (2015). Assessment of sublethal and transgenerational effects of Pirimicarb on the wheat aphids Rhopalosiphum padi and Sitobion avenae. PLoS ONE, 10(6), e0128936.
Acknowledgments
We thank our technical staff, Aya Kitahara, for her support for this study. We thank Dr. Makio Takeda for his support for this study. We thank Dr. Hiroyuki Mano for providing information about the bootstrap method in the R software. We thank Dr. Hiroaki Aoyama for helpful comments. We also thank Dr. Koji Satsuma for critiquing the manuscript. We thank Edanz Group (www.edanzediting.com/ac) and Editage (www.editage.jp) for editing the English language of this manuscript.
Author information
Authors and Affiliations
Contributions
All of the authors contributed to the study conception and design. Data collection was performed by Makoto Ishimota. Material preparation and data analysis were performed by Makoto Ishimota, Risako Tajiki-Nishino, Tomoki Fukuyama, and Masaki Sakamoto. The first draft of the manuscript was written by Makoto Ishimota, and all of the authors commented on revised versions of the manuscript. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Animals
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution. This article does not contain any studies with human participants or vertebrates performed by any of the authors.
Informed Consent
Informed consent was not required for this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 450 kb)
Rights and permissions
About this article
Cite this article
Ishimota, M., Tajiki-Nishino, R., Fukuyama, T. et al. Long-Term Tolerance Acquisition and Changes in Acetylcholinesterase Activity in Three Cladoceran Species After a 48-H Pulsed Exposure to Pirimicarb. Water Air Soil Pollut 231, 287 (2020). https://doi.org/10.1007/s11270-020-04670-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04670-3