Abstract
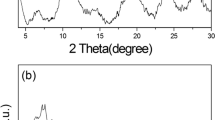
The effect of nickel in the adsorption of N-acetyl-p-aminophenol (paracetamol) on a metal-organic frameworks (MOFs) type MIL-101(Cr) was studied. The incorporation of Ni to MOFs adsorbent was carried out by impregnation and adjusted to give a 4.00 wt.% Ni. The adsorbents were characterized by specific surface area (SSA), surface acidity techniques, electrophoretic migration (EM), thermogravimetric analysis (TGA), and scanning electron microscope (SEM) and were tested by the adsorption of paracetamol solutions. The results showed that the Ni particles were well dispersed throughout the MIL-101(Cr) crystal increasing the acid strength and the density of acid site values in the MOFs surface. The increase in adsorption capacity of MIL-101(Cr) when Ni was incorporated can be attributed to the availability of metal atoms as adsorption centers that can adsorb the paracetamol by electronic retro-donation through π-type complexing.



Similar content being viewed by others
References
Ahmed, I., & Hwa, S. (2017). Applications of metal-organic frameworks in adsorption/separation processes via hydrogen bonding interactions. Chemical Engineering Journal, 310, 197–215.
Ahmed, M., & Theydan, S. (2012). Adsorption of cephalexin onto activated carbons from Albizia lebbeck seed pods by microwave-induced KOH and K2CO3 activations. Chemical Engineering Journal, 211, 200–207.
Andreozzi, R., Caprioa, V., Marotta, R., & Vognab, D. (2003). Paracetamol oxidation from aqueous solutions by means of ozonation and H2O2/UV system. Water Research, 37, 993–1004.
Aparicio, F., Camú, E., Villarroel, M., Escalona, N., & Baeza, P. (2013). Deep desulfurization by adsorption of 4,6-dimethyldibenzothiophene, study of adsorption on different transition metal oxides and supports. Journal of the Chilean Chemical Society, 58, 2057–2060.
Arikan, O. (2008). Degradation and metabolization of chlortetracycline during the anaerobic digestion of manure from medicated calves. Journal of Hazardous Materials, 158, 485–490.
Aslam, S., Subhan, F., Yan, Z., Etim, U. J., & Zeng, J. (2017). Dispersion of nickel nanoparticles in the cages of metal-organic framework: An efficient sorbent for adsorptive removal of thiophene. Chemical Engineering Journal, 315, 469–480.
Baeza, P., Aguila, G., Gracia, F., & Araya, P. (2008). Desulfurization by adsorption with copper supported on zirconia. Catalysis Communications, 9, 751–755.
Baeza, P., Aguila, G., Vargas, G., Ojeda, J., & Araya, P. (2012). Adsorption of thiophene and dibenzothiophene on highly dispersed Cu/ZrO2 adsorbents. Applied Catalysis B: Environmental, 111, 133–140.
Baeza, P., Aballay, P., Matus, C., Camú, E., Fernanda Ramirez, M., Eyzaguirre, J., & Ojeda, J. (2019). Degradation of paracetamol adsorbed on inorganic supports under UV irradiation. Water, Air, & Soil Pollution, 230, 34.
Benhmid, A., Edbey, K., Bukhzam, A., Alhowari, H., Mekhemer, G. A. H., & Zaki, M. I. (2018). Surface acidity of the supported molybdenum oxide catalysts probed by potentiometric titration of n-butylamine. International Research Journal of Pure & Applied Chemistry, 16, 1–7.
Cao, Y., Lu, S., Cui, W., Xu, Y., Cao, Z., & Zeng, Y. (2019). Adsorption desulfurization via π-complexation with Ag+-exchanged anionic metal–organic framework. Industrial & Engineering Chemistry Research, 58(16), 6704–6711.
Chen, T., Zhang, C., Qin, Y., Yang, H., Zhang, P., & Ye, F. (2017). Preparation of cationic mofs with mobile anions by anion stripping to remove 2,4-D from water. Materials, 10, 879.
Chen, W., & Huang, C. (2010). Adsorption and transformation of tetracycline antibiotics with aluminum oxide. Chemosphere, 79, 779–785.
Dantas, R., Contreras, S., Sans, C., & Esplugas, S. (2008). Sulfamethoxazole abatement by means of ozonation. Journal of Hazardous Materials, 150, 790.
De Andrade, J. R., Oliveira, M. F., Da Silva, M., & Vieira, M. (2018). Adsorption of pharmaceuticals from water and wastewater using nonconventional low-cost materials: A review. Industrial & Engineering Chemistry Research, 57(9), 3103–3127.
Dutta, M., Dutta, N., & Bhattacharya, K. (1999). Aqueous phase adsorption of certain beta-lactam antibiotics onto polymeric resins and activated carbon. Separation and Purification Technology, 16, 213–224.
Ersan, M., Bagda, E., & Bagda, E. (2013). Investigation of kinetic and thermodynamic characteristics of removal of tetracycline with sponge like, tannin based cryogels. Colloids and Surfaces B: Biointerfaces, 104, 75–82.
Fang, K., Ren, J., & Sun, Y. (2005). Effect of nickel precursors on the performance of Ni/AlMCM-41 catalysts for n-dodecane hydroconversion. Journal of Molecular Catalysis A: Chemical, 229(1), 51–58.
Férey, G., Mellot-Draznieks, C., Serre, C., Millange, F., Dutour, J., Surblé, S., & Margiolaki, I. (2005). A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science, 309, 2040–2042.
Furukawa, S., Reboul, J., Diring, S., Sumida, K., & Kitagawa, S. (2014). Structuring of metal–organic frameworks at the mesoscopic/macroscopic scale. Chemical Society Reviews, 43, 5700–5734.
Gao, J., & Pedersen, J. (2005). Adsorption of sulfonamide antimicrobial agents to clay minerals. Environmental Science & Technology, 39, 9509–9516.
Gao, Y., Liu, K., Kang, R., Xia, J., Yu, G., & Deng, S. (2018). A comparative study of rigid and flexible MOFs for the adsorption of pharmaceuticals: Kinetics, isotherms and mechanisms. Journal of Hazardous Materials, 359, 248–257.
Gobara, H. M. (2012). Characterization and catalytic activity of NiO/mesoporous aluminosilicate AlSBA-15 in conversion of some hydrocarbons. Egyptian Journal of Petroleum, 21(1), 1–10.
Guo, W., Shi, Y., Wang, H., Yang, H., & Zhang, G. (2010). Intensification of sonochemical degradation of antibiotics levofloxacin using carbon tetrachloride. Ultrasonics Sonochemistry, 17, 680–684.
Hasan, Z., & Hwa, S. (2015). Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. Journal of Hazardous Materials, 283, 329–339.
Hasan, Z., Choi, E.-J., & Jhung, S. H. (2013). Adsorption of naproxen and clofibric acid over a metal−organic framework MIL-101 functionalized with acidic and basic groups. Chemical Engineering Journal, 219, 537–544.
Hasan, Z., Khan, N. A., & Jhung, S. H. (2016). Adsorptive removal of diclofenac sodium from water with Zr-based metal−organic frameworks. Chemical Engineering Journal, 284, 1406–1413.
Herbst, A., Khutia, A., & Janiak, C. (2014). Brønsted instead of Lewis acidity in functionalized mil-101cr Mofs for efficient heterogeneous (nano-MOF) catalysis in the condensation reaction of aldehydes with alcohols. Inorganic Chemistry, 53(14), 7319–7333.
Hernandez-Maldonado, A. J., & Yang, R. T. (2003). Desulfurization of liquid fuels by adsorptionvia π-complexation with Cu(I)-Y and Ag-Y zeolites. Industrial Engineering Chemistry Research, 42, 123–129.
Hernández-Maldonado, A. J., & Yang, R. T. (2004). Desulfurization of diesel fuels via π-complexation with nickel(II)-exchanged X-and Y-zeolites. Industrial Engineering Chemistry Research, 43(4), 1081–1089.
Hong, D., Hwang, Y. K., Serre, C., Férey, G., & Chang, J. (2009). Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites: Surface functionalization, encapsulation, sorption and catalysis. Advances Functional Materials, 19, 1537–1552.
Janiak, C., & Vieth, J. (2010). MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New Journal of Chemistry, 34, 2366–2388.
Jara, C., Fino, D., Specchia, V., Saracco, G., & Spinelli, P. (2007). Electrochemical removal of antibiotics from wastewaters. Applied Catalalisys B: Environmental, 70, 479–487.
Ji, L., Chen, W., Duan, L., & Zhu, D. (2009). Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environmental Science and Technology, 43, 2322–2327.
Jin, J., Yang, Z., Xiong, W., Zhou, Y., Xu, R., Zhang, Y., & Zhou, C. (2019). Cu and Co nanoparticles co-doped MIL-101 as a novel adsorbent for efficient removal of tetracycline from aqueous solutions. Science of the Total Environment, 650, 408–418.
Karmakar, S., Roy, D., Janiak, C., & De, S. (2019). Insights into multi-component adsorption of reactive dyes on MIL-101-Cr metal organic framework: Experimental and modeling approach. Separation and Purification Technology, 215, 259–275.
Khan, N. A., & Jhung, S. H. (2017). Adsorptive removal and separation of chemicals with metal-organic frameworks: Contribution of π-complexation. Journal of Hazardous Materials, 325, 198–213.
La Farré, M., Pérez, S., Kantiani, L., & Barcelo, D. (2008). Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. Trends in Analytical Chemistry, 27, 991–1007.
Ma, J., Yu, F., Zhou, L., Jin, L., Yang, M., Luan, J., Tang, Y., Fan, H., & Yuan, Z. (2012). Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Applied Materials & Interfaces, 4, 5749–5760.
Mahmood, T., Saddique, M. T., Naeem, A., Westerhoff, P., Mustafa, S., & Alum, A. (2011). Comparison of different methods for the point of zero charge determination of NiO. Industrial Engineering Chemistry Research, 50, 10017–10023.
Navalon, S., Alvaro, M., & Garcia, H. (2008). Reaction of chlorine dioxide with emergent water pollutants: Product study of the reaction of three β-lactam antibiotics with ClO2. Water Research, 42, 1935–1942.
Peterson, J., Petrasky, L., Seymour, M., Burkhart, R., & Schuiling, A. (2012). Adsorption and breakdown of penicillin antibiotic in the presence of titanium oxide nanoparticles in water. Chemosphere, 87, 911–917.
Phong, V. H. N., Le, G. K., Hong Nguyen, T. M., Bui, X.-T., Nguyen, K. H., Rene, E. R., & Mohan, R. (2019). Acetaminophen micropollutant: Historical and current occurrences, toxicity, removal strategies and transformation pathways in different environments. Chemosphere, 236, 124391.
Putra, E., Pranowo, R., Sunarso, J., Indraswati, N., & Ismadji, S. (2009). Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Research, 43, 2419–2430.
Qin, F. X., Jia, S. Y., Liu, Y., Li, H. Y., & Wu, S. H. (2015). Adsorptive removal of bisphenol from aqueous solution using metal-organic frameworks. Desalination and Water Treatment, 54, 93–102.
Raven, P. H., Johnson, G. B., Mason, K. A., Losos, J. B., and Singer, S. R. (2014). The nature of molecules and properties of water. In Biology (10th ed., AP ed., pp. 17-30). New York: McGraw-Hill.
Rivera-Jiménez, S., & Hernández-Maldonado, A. (2008). Nickel(II) grafted MCM-41: A novel sorbent for the removal of naproxen from water. Microporous and Mesoporous Materials, 116, 246–252.
Seo, P. W., Khan, N. A., & Jhung, S. H. (2017). Removal of nitroimidazole antibiotics from water by adsorption over metal−organic frameworks modified with urea or melamine. Chemical Engineering Journal, 315, 92–100.
Su, S., Guo, W., Yi, C., Leng, Y., & Ma, Z. (2012). Degradation of amoxicillin in aqueous solution using sulphate radicals under ultrasound irradiation. Ultrasonics Sonochemistry, 19, 469–474.
Villaescusa, I., Fiol, N., Poch, J., Bianchi, A., & Bazzicalupi, C. (2011). Mechanism of paracetamol removal by vegetable wastes: The contribution of π–π interactions, hydrogen bonding and hydrophobic effect. Desalination, 270, 135–142.
Wang, T., Li, X., Dai, W., Fang, Y., & Huang, H. (2015). Enhanced adsorption of dibenzothiophene with zinc/copper-based metal-organic frameworks. Journal of Materials Chemistry A, 3, 21044–21050.
Xie, L., Liu, S., Han, Z., Jiang, R., Liu, H., Zhu, F., Zeng, F., Su, C., & Ouyang, G. (2015). Preparation and characterization of metal-organic framework MIL-101(Cr)-coated solid-phase microextraction fiber. Analytica Chimica Acta, 853, 303–310.
Xiong, W., Zeng, Z., Li, X., Zeng, G., Xiao, R., Yang, Z., & Qin, L. (2019). Ni-doped MIL-53(Fe) nanoparticles for optimized doxycycline removal by using response surface methodology from aqueous solution. Chemosphere, 232, 186–194.
Xu, L., Pan, J., Dai, J., Li, X., Hang, H., Cao, Z., & Yan, Y. (2012). Preparation of thermal-responsive magnetic molecularly imprinted polymers for selective removal of antibiotics from aqueous solution. Journal of Hazardous Materials, 233, 48–56.
Yu, F., Ma, J., Wang, J., Zhang, M., & Zheng, J. (2016). Magnetic iron oxide nanoparticles functionalized multi-walled carbon nanotubes for toluene, ethylbenzene and xylene removal from aqueous solution. Chemosphere, 146, 162–172.
Zhang, L. J., Li, F. Q., Ren, J. X., Ma, L. B., & Li, M. Q. (2018). Preparation of metal organic frameworks MIL-101 (Cr) with acetic acid as mineralizer. IOP Conference Series: Earth and Environmental Science, 199, 042038.
Zhang, X., Wu, F., Wu, X., Chen, P., & Deng, N. (2008). Photodegradation of acetaminophen in TiO2 suspended solution. Journal of Hazardous Materials, 157, 300–307.
Acknowledgments
The authors are grateful to DI – consolidado 039.369 – PUCV.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baeza, P., Astudillo, C., Diaz, M. et al. Effect of the Incorporation of Ni in the Adsorption Capacity of Paracetamol (N-Acetyl-P-Aminophenol) on MIL-101(Cr). Water Air Soil Pollut 231, 245 (2020). https://doi.org/10.1007/s11270-020-04584-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04584-0