Abstract
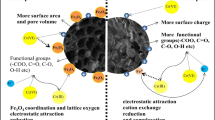
In this study, adsorption experiments on Cd(II) ions in aqueous solution by biochars pyrolyzed from millet bran (MBBC) at 400~800 °C were carried out and MBBC had superior adsorption performance at pyrolysis temperature of 600~800 °C. Then, biochars were modified by potassium permanganate (MBBC-PP), potassium ferrate (MBBC-PF), and citric acid (MBBC-CA). The results showed that the adsorption capacities of these adsorbents were in the order: MBBC-PP > MBBC-PF > MBBC-CA > MBBC. FT-IR, XRD, and Raman were used to determine the characteristics of biochars and explore the main adsorption mechanism causing by modification. These characterizations confirmed that manganese oxide and iron oxide particles were loaded on the surface of MBBC-PP and MBBC-PF, respectively. For MBBC-PP and MBBC-PF, the adsorption sites generated by the loaded metal oxides play more important role than other factors in Cd(II) adsorption. For MBBC-CA, surface functional groups were the main contributor during Cd(II) adsorption. Batch adsorption experiments demonstrate a Langmuir model fit for Cd(II). The sorption kinetics of Cd(II) on adsorbents follows pseudo-second-order kinetics. Modified biochars, especially the one modified by KMnO4, could act as effective alternatives to enhance heavy metals removal from aqueous solutions.




Similar content being viewed by others
References
Ahmad, M., Lee, S. S., Dou, X., Mohan, D., Sung, J. K., Yang, J. E., & Ok, Y. S. J. B. T. (2012). Effects of pyrolysis temperature on soybean Stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresource Technology, 118, 536–544.
Ahmed, M. B., Zhou, J. L., Ngo, H. H., Johir, M. A. H., Sun, L., Asadullah, M., & Belhaj, D. (2018). Sorption of hydrophobic organic contaminants on functionalized biochar: protagonist role of pi-pi electron-donor-acceptor interactions and hydrogen bonds. Journal of Hazardous Materials, 360, 270–278.
Al-Wabel, M.I., Al-Omran, A., El-Naggar, A.H., Nadeem, M., & Usman, A.R.A.J.B.T. (2013). Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. 131, 374.
Bagchi, D., Maji, T.K., Sardar, S., Lemmens, P., Bhattacharya, C., Karmakar, D. & Pal, S.K.J.P.C.C.P.P (2017). Sensitized ZnO nanorod assemblies to detect heavy metal contaminated phytomedicines: spectroscopic and simulation studies. 19, 2503.
Barakat, M.A.J.A.J.O.C. (2011). New trends in removing heavy metals from industrial wastewater. 4, 361–377.
Chen, B., Chen, Z. & Lv, S.J.B.T. (2011). A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. 102, 716–723.
Chen, Q., Zheng, J. W., Zheng, L. C., Dang, Z., & Zhang, L. J. (2018). Classical theory and electron-scale view of exceptional Cd(II) adsorption onto mesoporous cellulose biochar via experimental analysis coupled with DFT calculations. Chemical Engineering Journal, 350, 1000–1009.
Demirbas, A. J. J.O.H.M. (2008). Heavy metal adsorption onto agro-based waste materials: a review. 157, 220–229.
El-Banna, M. F., Mosa, A., Gao, B., Yin, X., Ahmad, Z., & Wang, H. (2018). Sorption of lead ions onto oxidized bagasse-biochar mitigates Pb-induced oxidative stress on hydroponically grown chicory: experimental observations and mechanisms. Chemosphere, 208, 887–898.
Fan, Z., Zhang, Q., Gao, B., Li, M., Liu, C., & Qiu, Y. (2018). Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere, 217, 85–94.
Fu, F. & Wang, Q. J. J.O.E.M. (2011). Removal of heavy metal ions from wastewaters: a review. 92, 407–418.
Inyang, M., Gao, B., Yao, Y., Xue, Y., Zimmerman, A.R., Pullammanappallil, P. & Cao, X.J.B.T. (2012). Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. 110, 50–56.
Jiang, B., Lin, Y., & Mbog, J. C. (2018). Biochar derived from swine manure digestate and applied on the removals of heavy metals and antibiotics. Bioresource Technology, 270, 603–611.
Jung, K.W., Choi, B.H., Jeong, T.U. & Ahn, K.H.J.B.T.T. (2016). Facile synthesis of magnetic biochar/Fe3O4 nanocomposites using electro-magnetization technique and its application on the removal of acid orange 7 from aqueous media. 220, 672–676.
Kim, K.H., Kim, J.Y., Cho, T.S., & Choi, J.W.J.B.T. (2012). Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). 118, 158.
Kim, W.K., Shim, T., Kim, Y.S., Hyun, S., Ryu, C., Park, Y.K. & Jung, J.J.B.T. (2013). Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. 138, 266–270.
Li, L., Quinlivan, P.A., & Knappe, D.R.U.J.C. (2002). Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. 40, 2085–2100.
Li, B., Yang, L., Wang, C. Q., Zhang, Q.P., Liu, Q.C., Li, Y.D. & Xiao, R.J.C. (2017a). Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. 175, 332.
Li, H., Dong, X., Silva, E.B.D., Oliveira, L.M.D., Chen, Y. & Ma, L.Q.J.C. (2017c). Mechanisms of metal sorption by biochars: biochar characteristics and modifications. 178, 466–478.
Narzari, R., Bordoloi, N., Sarma, B., Gogoi, L., Gogoi, N., Borkotoki, B., & Kataki, R. (2017). Fabrication of biochars obtained from valorization of biowaste and evaluation of its physicochemical properties. Bioresource Technology, 242, 324–328.
Ni, B. J., Huang, Q. S., Wang, C., Ni, T. Y., Sun, J., & Wei, W. (2018). Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere, 219, 351–357.
Paethanom, A., & Yoshikawa, K.J.E. (2012). Influence of pyrolysis temperature on rice husk char characteristics and its tar adsorption capability. 5, 4941–4951.
Peng, H., Gao, P., Chu, G., Pan, B., Peng, J. & Xing, B.J.E.P. (2017). Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. 229.
Rajapaksha, A.U., Chen, S.S., Tsang, D.C.W., Zhang, M., Vithanage, M., Mandal, S., Gao, B., Bolan, N.S., & Yong, S.O.J.C. (2016). Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification. 148, 276–291.
Regmi, P., Garcia Moscoso, J.L., Kumar, S., Cao, X., Mao, J., & Schafran, G.J.J.O.E.M. (2012). Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. 109, 61–69.
Shan, D., Deng, S., Zhao, T., Wang, B., Wang, Y., Huang, J., Gang, Y., Winglee, J. & Wiesner, M.R.J.J.O.H.M. (2015). Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. 305, 156.
Shinogi, Y., & Kanri, Y.J.B.T. (2003). Pyrolysis of plant, animal and human waste: physical and chemical characterization of the pyrolytic products. 90, 241.
Sizmur, T., Quilliam, R., Puga, A. P., Morenojimnez, E., Beesley, L., & Gomezeyles, J.L. (2015). Application of Biochar for Soil Remediation.
Sun, L., Chen, D., Wan, S. & Yu, Z.J.B.T. (2015). Peerformance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. 198, 300–308.
Vafakhah, S., Bahrololoom, M. E., Bazarganlari, R. & Saeedikhani, M. J. J.O.E.C.E. (2014). Removal of copper ions from electroplating effluent solutions with native corn cob and corn stalk and chemically modified corn stalk. 2, 356–361.
Wan, Z., Li, M., Zhang, Q., Fan, Z., Verpoort, F.J.E.S., & Research, P. (2018). Concurrent reduction-adsorption of chromium using m-phenylenediamine-modified magnetic chitosan: kinetics, isotherm, and mechanism. pp. 1–12.
Wang, Y., Wang, X., Wang, X., Liu, M., Yang, L., Wu, Z., Xia, S., Zhao, J.J.C., Physicochemical, S.A., & Aspects, E. (2012). Adsorption of Pb(II) in aqueous solutions by bamboo charcoal modified with KMnO4 via microwave irradiation. 414, 1–8.
Wang, H., Gao, B., Wang, S., Fang, J., Xue, Y. & Yang, K.J.B.T. (2015). Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. 197, 356–362.
Won, S. W., Kotte, P., Wei, W., Lim, A. & Yun, Y. S. J. B.T. (2014). Biosorbents for recovery of precious metals. 160, 203–212.
Xiang, J., Lin, Q., Cheng, S., Guo, J., Yao, X., Liu, Q., Yin, G., Liu, D.J.E.S., & Research, P. (2018). Enhanced adsorption of Cd(II) from aqueous solution by a magnesium oxide? rice husk biochar composite. pp. 1–11.
Xin, H., Ding, Z.H., Zimmerman, A. R., Wang, S.S., & Gao, B.J.W.R. (2015). Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. 68, 206–216.
Xu, Y., Liu, Y., Liu, S., Tan, X., Zeng, G., Zeng, W., Ding, Y., Cao, W., & Zheng, B. (2016). Enhanced adsorption of methylene blue by citric acid modification of biochar derived from water hyacinth (Eichornia crassipes). Environmental Science and Pollution Research International, 23, 23606–23618.
Yang, E., Yao, C., Liu, Y., Zhang, C., Jia, L., Li, D., Fu, Z., Sun, D., Kirk, S.R. & Yin, D.J.F. (2018). Bamboo-derived porous biochar for efficient adsorption removal of dibenzothiophene from model fuel. 211, 121–129.
Yuan, J.H., Xu, R.K. & Zhang, H.J.B.T. (2011). The forms of alkalis in the biochar produced from crop residues at different temperatures. 102, 3488–3497.
Zhang, X., Fu, W., Yin, Y., Chen, Z., Qiu, R., Simonnot, M. O., & Wang, X. (2018). Adsorption-reduction removal of Cr(VI) by tobacco petiole pyrolytic biochar: batch experiment, kinetic and mechanism studies. Bioresource Technology, 268, 149–157.
Zhou, Y., Gao, B., Zimmerman, A. R., Chen, H., Zhang, M. & Cao, X.J.B.T. (2014). Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. 152, 538.
Funding
This work was financially supported by the Fundamental Research Funds for the Central Universities (No. 185206002, No. 201809706019) and the Science and Technology Infrastructure Program of Hubei Province of China (No. 2015BCA304).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 478 kb)
Rights and permissions
About this article
Cite this article
Qiu, Y., Zhang, Q., Li, M. et al. Adsorption of Cd(II) From Aqueous Solutions by Modified Biochars: Comparison of Modification Methods. Water Air Soil Pollut 230, 84 (2019). https://doi.org/10.1007/s11270-019-4135-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4135-8