Abstract
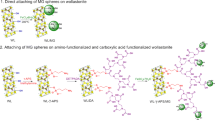
The contamination of drinking water with arsenic has been a problem in a lot of countries around the world because of its toxicological and carcinogenic effects on human health. Porous materials modified with Fe3O4 nanoparticles (Fe3O4 NPs) represent convenient removers for that contaminant. A co-precipitation method of Fe(III) and Fe(II) in alkaline media was applied to obtain Fe3O4 NPs. In a first stage, single nanoparticles were synthesized and stabilized with carboxylic acids. A characterization with attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), Raman spectroscopy, and X-ray diffraction (XRD) confirms a magnetite-type structure. Moreover, transmission electron microscopy (TEM) and calculations from XRD data using Scherrer’s equation indicate an average particle size of 13 nm and an average crystallite size of 10 nm, both independent of the stabilizer used. Then, the co-precipitation method studied was applied to modify kaolin, bentonite, diatomite, and silica and thus prepare magnetic composites having support-magnetite weight ratios of 2:1. Among them, silica-modified material presented the best hydraulic characteristics, an important aspect for large-scale applications such as removal under gravity. This composite has the capacity to remove up to 80 and 70% for initial concentrations of 25 and 50 μg/L, respectively, representing a convenient remover for processes developed in subsequent stages or in continuous flow.










Similar content being viewed by others
References
Ahoulé, D. G., Lalanne, F., Mendret, J., Brosillon, S., & Maïga, A. H. (2015). Arsenic in African waters: a review. Water, Air, and Soil Pollution. doi:10.1007/s11270-015-2558-4.
Akin, I., Arslan, G., Tor, A., Ersoz, M., & Cengeloglu, Y. (2012). Arsenic(V) removal from underground water by magnetic nanoparticles synthesized from waste red mud. Journal of Hazardous Materials. doi:10.1016/j.jhazmat.2012.06.024.
Angulo-Zamora, F. (2015). Vigesimoprimer Informe Estado de la Nación en Desarrollo Humano Sostenible: Informe final, Gestión del Recurso Hídrico y Saneamiento en Costa Rica. Programa Estado de la Nación. http://estadonacion.or.cr/files/biblioteca_virtual/021/ambiente/Angulo_RH_y_saneamiento.pdf.
Aphesteguy, J. C., Kurlyandskaya, G. V., De Celis, J. P., Safronov, A. P., & Schegoleva, N. N. (2015). Magnetite nanoparticles prepared by co-precipitation method in different conditions. Materials Chemistry and Physics. doi:10.1016/j.matchemphys.2015.05.044.
Astorga, Y. (2013). Decimonoveno Informe Estado de la Nación en Desarrollo Humano Sostenible: Informe final, Gestión del Recurso Hídrico. Programa Estado de la Nación. http://estadonacion.or.cr/files/biblioteca_virtual/019/astorga_2013.pdf.
Astorga, Y. & Angulo, F. (2013). Vigésimo Informe Estado de la Nación en Desarrollo Humano Sostenible: Informe final, Gestión del Recurso Hídrico y Saneamiento. Programa Estado de la Nación. http://estadonacion.or.cr/files/biblioteca_virtual/020/ambiente/Astorga%20y%20Angulo_Recurso%20H%C3%ADdrico%20y%20Saneamiento.pdf.
Baig, S. A., Sheng, T., Sun, C., Xue, X., Tan, L., & Xu, X. (2014). Arsenic removal from aqueous solutions using Fe3O4-HBC composite: effect of calcination on adsorbents performance. PloS One, 9(6), e100704. doi:10.1371/journal.pone.0100704.
Brandhuber, P., & Amy, G. (2001). Arsenic removal by a charged ultrafiltration membrane—influences of membrane operating conditions and water quality on arsenic rejection. Desalination. doi:10.1016/S0011-9164(01)00350-2.
Castro de Esparza, M. L. (2008). The presence of arsenic in drinking water in Latin America and its effect on public health. In: J. Bundschuh, M. A. Armienta, P. Birkle, P. Bhattacharya, J. Matschullat, & A. B. Mukherjee (Ed.), Natural arsenic in groundwaters of Latin America. Boca Raton: CRC Press.
Chen, H., Liu, L., Gong, R., Wei, R., Yi, Q., & Qiu, A. (2016). Comparison of kinetics of arsenic(V) adsorption on two types of red soil weathered from granite and sandstone. Water, Air, & Soil Pollution. doi:10.1007/s11270-016-3107-5.
Cheng, Y., Tan, R., Wang, W., Guo, Y., Cui, P., & Song, W. (2010). Controllable synthesis and magnetic properties of Fe3O4 and Fe3O4@SiO2 microspheres. Journal of Materials Science. doi:10.1007/s10853-010-4583-4.
Choong, T. S. Y., Chuah, T. G., Robiah, Y., Gregory Koay, F. L., & Azni, I. (2007). Arsenic toxicity, health hazards and removal techniques from water: an overview. Desalination. doi:10.1016/j.desal.2007.01.015.
Cumbal, L., German, M., Sengupta, A., & Shengheng, H. (2014). Robust and reusable hybrid nanosorbent to mitigate arsenic crisis: from laboratory to masses in the field. In: M. I. Litter, H. B. Nicolli, M. Meichtry, N. Quici, J. Bundschuh, P. Bhattacharya, & R. Naidu (Ed.), One century of the discovery of arsenicosis in Latin America (1914–2014) (pp. 797–801). Boca Raton: CRC Press.
Devi, R. R., Umlong, I. M., Das, B., Borah, K., Thakur, A. J., Raul, P. K., Banerjee, S., & Singh, L. (2014). Removal of iron and arsenic (III) from drinking water using iron oxide-coated sand and limestone. Applied Water Science. doi:10.1007/s13201-013-0139-5.
Dixit, S., & Hering, J. (2003). Comparison of arsenic (V) and arsenic (III) sorption onto iron oxide minerals: implications for arsenic mobility. Environmental Science & Technology. doi:10.1021/es030309t.
Elcik, H., Cakmakci, M., Sahinkaya, E., & Ozkaya, B. (2013). Arsenic removal from drinking water using low pressure membranes. Industrial & Engineering Chemistry Research. doi:10.1021/ie401393p.
Gao, M., Li, W., Dong, J., Zhang, Z., & Yang, B. (2011). Synthesis and characterization of superparamagnetic Fe3O4@SiO2 core-shell composite nanoparticles. World Journal of Condensed Matter Physics. doi:10.4236/wjcmp.2011.12008.
Giménez, J., Martínez, M., de Pablo, J., Rovira, M., & Duro, L. (2007). Arsenic sorption onto natural hematite, magnetite, and goethite. Journal of Hazardous Materials. doi:10.1016/j.jhazmat.2006.07.020.
Gnanaprakash, G., Philip, J., Jayakumar, T., & Raj, B. (2007). Effect of digestion time and alkali addition rate on physical properties of magnetite nanoparticles. Journal of Physical Chemistry B. doi:10.1021/jp071299b.
Herrera, J. (2016). Vigesimosegundo Informe Estado de la Nación en Desarrollo Humano Sostenible: Informe final, Recurso Hídrico y Saneamiento: Avances y Desafíos. Programa Estado de la Nación. http://estadonacion.or.cr/files/biblioteca_virtual/022/Ambiente/Herrera_J_2016Recursos_hidricos.pdf.
Hu, X., Ding, Z., Zimmerman, A. R., Wang, S., & Gao, B. (2015). Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Research. doi:10.1016/j.watres.2014.10.009.
Iida, H., Takayanagi, K., Nakanishi, T., & Osaka, T. (2007). Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. Journal of Colloid and Interface Science. doi:10.1016/j.jcis.2007.05.047.
Jain, A., & Loeppert, R. H. (2000). Effect of competing anions on the adsorption of arsenate and arsenite by ferrihydrite. Journal of Environmental Quality. doi:10.2134/jeq2000.00472425002900050008x.
Kartinen, E. O., & Martin, C. J. (1995). An overview of arsenic removal processes. Desalination. doi:10.1016/j.desal.2004.07.031.
Lata, S., & Samadder, S. R. (2016). Removal of arsenic from water using nano adsorbents and challenges: a review. Journal of Environmental Management. doi:10.1016/j.jenvman.2015.10.039.
Maiti, S. (2014). Arsenic-induced mutagenesis and carcinogenesis: a possible mechanism. In S. J. S. Flora (Ed.), Handbook of arsenic toxicology (pp. 233–279). Cambridge: Academic Press.
Mamindy-Pajany, Y., Hurel, C., Marmier, N., & Roméo, M. (2011). Arsenic (V) adsorption from aqueous solution onto goethite, hematite, magnetite and zero-valent iron: effects of pH, concentration and reversibility. Desalination. doi:10.1016/j.desal.2011.07.046.
Mandal, B. K. (2014). Changing concept of arsenic toxicity with development of speciation techniques. In S. J. S. Flora (Ed.), Handbook of arsenic toxicology (pp. 179–201). Cambridge: Academic Press.
Mascolo, M. C., Pei, Y., & Ring, T. A. (2013). Room temperature co-precipitation synthesis of magnetite nanoparticles in a large ph window with different bases. Materials. doi:10.3390/ma6125549.
Mayo, J. T., Yavuz, C., Yean, S., Cong, L., Shipley, H., Yu, W., Falkner, J., Kan, A., Tomson, M., & Colvin, V. L. (2007). The effect of nanocrystalline magnetite size on arsenic removal. Science and Technology of Advanced Materials. doi:10.1016/j.stam.2006.10.005.
Miller, J. N., & Miller, J. C. (2010) Statistics and chemometrics for analytical chemistry, 6th ed. (pp. 124–126). London: Pearson.
Mishra, T., & Mahato, D. K. (2016). A comparative study on enhanced arsenic(V) and arsenic(III) removal by iron oxide and manganese oxide pillared clays from ground water. Journal of Environmental Chemical Engineering. doi:10.1016/j.jece.2016.01.022.
Montero-Campos, V., & Puente-Urbina, A. (2016). Continuous-flow removal of arsenic in drinking water by filtering down through Fe3O4@SiO2 magnetic composite. Journal of Water Resource and Protection. doi:10.4236/jwarp.2016.85051.
Montero-Campos, V., Quesada-Kimsey, J., Ledezma-Espinoza, A., & Sandoval-Mora, J. (2010). Determinación de arsénico en abastecimientos de agua para consumo humano de la provincia de Cartago, Costa Rica. Acta Médica Costarricense, 52(2), 96–101.
Mora-Alvarado, D. A. (2013). Agua para consumo humano: Estudio comparativo de la contaminación por arsénico en Argentina, Chile y los países de Centroamerica. Tres Ríos: Acueductos y Alcantarillados.
Mora-Alvarado, D. A. (2014). La problemática del arsénico: Costa Rica en el contexto Latinoamericano. Acueductos y Alcantarillados. Tres Ríos: Acueductos y Alcantarillados.
Ngomsik, A. F., Bee, A., Draye, M., Cote, G., & Cabuil, V. (2005). Magnetic nano- and microparticles for metal removal and environmental applications: a review. Comptes Rendus Chimie. doi:10.1016/j.crci.2005.01.001.
Oliveira, L. C. A., Rios, R. V. R. A., Fabris, J. D., Sapag, K., Garg, V. K., & Lago, R. M. (2003). Clay-iron oxide magnetic composites for the adsorption of contaminants in water. Applied Clay Science. doi:10.1016/S0169-1317(02)00156-4.
Oliveira, L. C., Petkowicz, D. I., Smaniotto, A., & Pergher, S. B. C. (2004). Magnetic zeolites: a new adsorbent for removal of metallic contaminants from water. Water Research. doi:10.1016/j.watres.2004.06.008.
Onishi, H. (1969). Arsenic. In K. H. Wedepohl (Ed.), Handbook of geochemistry (chapter 33). New York: Springer-Verlag.
Petcharoen, K., & Sirivat, A. (2012). Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Materials Science and Engineering: B. doi:10.1016/j.mseb.2012.01.003.
Puente-Urbina, A., Hollenbach, J., Céspedes-Camacho, I. F., Matysik, J., & Valle-Bourrouet, G. (2016). Effect of pretreatment temperature on the surface modification of diatomite with trimethylchlorosilane. Journal of Porous Materials. doi:10.1007/s10934-016-0204-1.
Qin, C., Liu, L., Han, Y., & Chen, C. (2016). Mesoporous magnetic ferrum-yttrium binary oxide: a novel adsorbent for efficient arsenic removal from aqueous solution. Water, Air, & Soil Pollution. doi:10.1007/s11270-016-3032-7.
Raven, K. P., Jain, A., & Loeppert, R. H. (1998). Arsenite and arsenate adsorption on ferrihydite: kinetics, equilibrium, and adsorption envelopes. Environmental Science and Technology. doi:10.1021/es970421p.
Rojas-Chaves, P., Vargas-Benavides, M. J., Araya-Obando, A., Valverde-Cerdas, J., & Romero-Esquivel, L. G. (2015). Estudio de remoción de arsénico en agua potable a nivel domiciliar mediante oxidación solar y coagulación-floculación. Tecnología en Marcha. doi:10.18845/tm.v28i4.2443.
Romero, L. G., Valverde, J., Rojas, P., Vargas, MJ, & Araya, J. A. (2014). Exploring low-cost arsenic removal alternatives in Costa Rica. In: M. I. Litter, H. B. Nicolli, M. Meichtry, N. Quici, J. Bundschuh, P. Bhattacharya, & R. Naidu (Ed.), One century of the discovery of arsenicosis in Latin America (1914–2014) 853–855. Boca Raton: CRC Press.
Saiz, J., Bringas, E., & Ortiz, I. (2014). Functionalized magnetic nanoparticles as new adsorption materials for arsenic removal from polluted waters. Journal of Chemical Technology & Biotechnology. doi:10.1002/jctb.4331.
Salem Attia, T. M., Hu, X. L., & Yin, D. Q. (2013). Synthesized magnetic nanoparticles coated zeolite for the adsorption of pharmaceutical compounds from aqueous solution using batch and column studies. Journal of Experimental Nanoscience. doi:10.1016/j.chemosphere.2013.07.046.
Schwarzenbach, R. P., Egli, T., Hofstetter, T. B., von Gunten, U., & Wehrli, B. (2010). Global water pollution and human health. Annual Review of Environment and Resources. doi:10.1146/annurev-environ-100809-125342.
Shebanova, O. N., & Lazor, P. (2003). Raman spectroscopic study of magnetite (FeFe2O4): a new assignment for the vibrational spectrum. Journal of Solid State Chemistry. doi:10.1016/S0022-4596(03)00294-9.
Shen, L., Qiao, Y., Guo, Y., Meng, S., Yang, G., Wu, M., & Zhao, J. (2014). Facile co-precipitation synthesis of shape-controlled magnetite nanoparticles. Ceramics International. doi:10.1016/j.ceramint.2013.07.037.
Shipley, H. J., Yean, S., Kan, A. T., & Tomson, M. B. (2009). Adsorption of arsenic to magnetite nanoparticles: effect of particle concentration, pH, ionic strength, and temperature. Environmental Toxicology and Chemistry. doi:10.1897/08-155.1.
Slavov, L., Abrashev, M. V., Merodiiska, T., Gelev, C., Vandenberghe, R. E., Markova-Deneva, I., & Nedkov, I. (2010). Raman spectroscopy investigation of magnetite nanoparticles in ferrofluids. Journal of Magnetism and Magnetic Materials. doi:10.1016/j.jmmm.2010.01.005.
Smedley, P. L., & Kinniburg, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry. doi:10.1016/S0883-2927(02)00018-5.
Starbird-Pérez, R., & Montero-Campos, V. (2015). Synthesis of magnetic iron oxide nanoparticles toward arsenic removal from drinking water. Tecnología en Marcha. doi:10.18845/tm.v28i3.2410.
Thirunavukkarasu, O. S., Viraraghavan, T., & Subramanian, K. S. (2003). Arsenic removal from drinking water using iron oxide-coated sand. Water, Air, & Soil Pollution. doi:10.1023/A:1022073721853.
Valenzuela, R., Fuentes, M. C., Parra, C., Baeza, J., Duran, N., Sharma, S. K., Knobel, M., & Freer, J. (2009). Influence of stirring velocity on the synthesis of magnetite nanoparticles (Fe3O4) by the co-precipitation method. Journal of Alloys and Compounds. doi:10.1016/j.jallcom.2009.08.087.
Viraraghavan, T., Subramanian, K. S., & Aruldoss, J. A. (1999). Arsenic in drinking water—problems and solutions. Water Science and Technology. doi:10.1016/S0273-1223(99)00432-1.
Wan, J., Tang, G., & Qian, Y. (2007). Room temperature synthesis of single-crystal Fe3O4 nanoparticles with superparamagnetic property. Applied Physics A: Materials Science and Processing. doi:10.1007/s00339-006-3766-y.
Wang, J., Xu, W., Chen, L., Huang, X., & Liu, J. (2014). Preparation and evaluation of magnetic nanoparticles impregnated chitosan beads for arsenic removal from water. Chemical Engineering Journal. doi:10.1016/j.cej.2014.04.061.
Wang, Y., Wang, S., Wang, X., Zhang, Z., & Jia, Y. (2016). Adsorption behavior and removal mechanism of arsenic from water by Fe (III)-modified 13X molecular sieves. Water, Air, & Soil Pollution. doi:10.1007/s11270-016-2955-3.
Yavuz, C. T., Mayo, J. T., Yu, W. W., Prakash, A., Falkner, J. C., Yean, S., Cong, L., Shipley, H. J., Kan, A., Tomson, M., Natelson, D., & Colvin, V. L. Monodisperse low-field magnetic separation of Fe3O4 nanocrystals. Science. doi:10.1126/science.1131475.
Yean, S., Cong, L., Yavuz, C. T., Mayo, J. T., Yu, W. W., Kan, A. T., Colvin, V. L., & Tomson, M. B. (2005). Effect of magnetite particle size on adsorption and desorption of arsenite and arsenate. Journal of Materials Research. doi:10.1557/jmr.2005.0403.
Acknowledgements
The authors thank the Vicerrectoría de Investigación y Extensión (Grant: 5402-1460-8101) for supporting the research reported in this article. Thanks are also given to Ana Lucía Moya for her support in synthesis and characterization of magnetic nanoparticles, Marit Möller for performing removal tests and the German Academic Exchange Service (DAAD) for financing her work in the frame of the Research Internships in Science and Engineering program, Ana Victoria Cubero for her support in Atomic Absorption Spectroscopy analyses, Luis Fernando Alvarado for TEM measurements, Dr.-Ing. Teodolito Guillén-Girón and Esteban Hernández for XRD analyses, and Dr. Dionisio Gutiérrez-Fallas and Dr. Ernesto Montero-Zeledón for Raman spectroscopy analyses. Dr. Jorge Cubero-Sesín is acknowledged for helpful discussions of XRD results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puente-Urbina, A., Montero-Campos, V. Porous Materials Modified with Fe3O4 Nanoparticles for Arsenic Removal in Drinking Water. Water Air Soil Pollut 228, 374 (2017). https://doi.org/10.1007/s11270-017-3513-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3513-3