Abstract
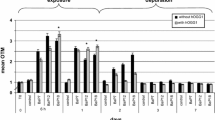
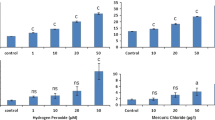
DNA damage and oxidative stress in marine gastropod Morula granulata was measured after in vivo exposure to four different concentrations (10, 25, 50, and 100 μg/L) of phenanthrene. Comet assay was used for measurement of DNA damage, whereas oxidative stress was assessed using a battery of biomarkers such as glutathione-S-transferase (GST), catalase (CAT), and lipid peroxidation (LPO). Our data showed concentration-dependent increase in percentage DNA in tail (TDNA), LPO, and GST activity in gastropods exposed to phenanthrene. CAT activity in gastropods was not found to be consistent with the phenanthrene concentrations. Significant increase in TDNA was observed at all concentrations above 10 μg/L of phenanthrene. Positive correlations were observed among oxidative stress biomarker and TDNA. Integrated biomarker response (IBR) analysis showed that among the four biomarkers, LPO and DNA damage (TDNA) were the most sensitive in response to phenanthrene exposure. Our results clearly showed that phenanthrene is genotoxic to gastropods and also causes oxidative stress.



Similar content being viewed by others
References
Afsar, N., Siddiqui, G., & Ayub, Z. (2012). A record of imposex in Morula granulata (Mollusca: Gastropoda: Muricidae) from Pakistan. Pakistan Journal of Zoology, 44(2), 572–576.
Ali, D., Ali, H., Alarifi, S., Kumar, S., Serajuddin, M., Mashih, A. P., Ahmed, M., Khan, M., Adil, S. F., Shaik, M. R., & Ansari, A. A. (2015). Impairment of DNA in a freshwater gastropod (Lymnea luteola L.) after exposure to titanium dioxide nanoparticles. Archives of Environmental Contamination and Toxicology, 68, 543–552.
Almamoori, A. M. J., Salman, J. M., Hughes, A. R., & AL-Saadi, A. J. (2013). Molecular biomarkers in the clam (Corbicula Fluminea) and snail (Viviparus Bengalensis) induced by acute exposure to Zn and Pb. Global Journal of Bioscience Technology, 2(2), 135–138.
An, L., Zheng, B., Wang, L., Zhang, Y., Chen, H., Zhao, X., Zhang, L., & Lei, K. (2012). Biomarker responses and genotoxicity in the mud snail (Bullacta exarata) as indicators of coastal contamination. Marine Pollution Bulletin, 64, 303–309.
Anderson, D., Yu, T. W., Philips, B. J., & Schmerzer, P. (1994). The effect of various antioxidant and other modifying agents on oxygen radical-generated DNA damage in human lymphocytes in the comet assay. Mutation Research, 307, 261–271.
Angeletti, D., Sebbio, C., Carere, C., Cimmaruta, R., Nascetti, G., Pepe, G., & Mosesso, P. (2013). Terrestrial gastropods (Helix spp) as sentinels of primary DNA damage for biomonitoring purposes: a validation study. Environmental and Molecular Mutagenesis, 54, 204–212.
Bandowe, B. A., Bigalke, M., Boamah, L., Nyarko, E., Saalia, F. K., & Wilcke, W. (2014). Polycyclic aromatic compounds (PAHs and oxygenated PAHs) and trace metals in fish species from Ghana (West Africa): bioaccumulation and health risk assessment. Environment International, 65, 135–146.
Banni, M., Negri, A., Dagnino, A., Jebali, J., Ameur, S., & Boussetta, H. (2010). Acute effects of benzo[a]pyrene on digestive gland enzymatic biomarkers and DNA damage on mussel Mytilus galloprovincialis. Ecotoxicology and Environmental Safety, 73(5), 842–848.
Barda, I., Purina, I., Rimsa, E., & Balode, M. (2014). Seasonal dynamics of biomarkers in infaunal clam Macoma balthica from the Gulf of Riga (Baltic Sea). Journal of Marine System, 129, 150–156.
Bech, M. (2002). A survey of imposex in muricids from 1996 to 2000 and identification of optimal indicators of tributylin contamination along the east coast of Phuket Island, Thailand. Marine Pollution Bulletin, 44, 887–896.
Beliaeff, B., & Burgeot, T. (2002). Integrated biomarker response: a useful tool for ecological risk assessment. Environmental Toxicology and Chemistry, 21, 1316–1322.
Bhagat, J., Ingole, B., Sarkar, A., & Gunjikar, M. (2012). Measurement of DNA damage in Planaxis sulcatus as a biomarker of genotoxicity. The Ecoscan, 1(01–04), 219–223.
Boshoff, M., Jordaens, K., Backeljau, T., Lettens, S., Tack, F., Vandecasteele, B., De Jonge, M., & Bervoets, L. (2013). Organ- and species-specific accumulation of metals in two land snail species (Gastropoda, Pulmonata). The Science of the Total Environment, 449, 470–481.
Campillo, J. A., Albentosa, M., Valdés, N. J., Moreno-González, R., & León, V. M. (2013). Impact assessment of agricultural inputs into a Mediterranean coastal lagoon (Mar Menor, SE Spain) on transplanted clams (Ruditapes decussatus) by biochemical and physiological responses. Aquatic Toxicology, 142–143, 365–79.
Cheung, C. C. C., Zheng, G. J., Li, A. M. Y., Richardson, B. J., & Lam, P. K. S. (2001). Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquatic Toxicology, 52, 189–203.
Correia, A. D., Gonc¸ alves, R., Scholze, M., Ferreira, M., & Henriques, M. A. R. (2007). Bio-chemical and behavioral responses in gilthead seabream (Sparus aurata) to phenanthrene. Journal of Experimental Marine Biology and Ecology, 347, 109–122.
Dabrowska, H., Kopko, O., Turja, R., Lehtonen, K. K., Góra, A., Polak-Juszczak, L., Warzocha, J., & Kholodkevich, S. (2013). Sediment contaminants and contaminant levels and biomarkers in caged mussels (Mytilus trossulus) in the southern Baltic Sea. Marine Environmental Research, 84, 1–9. doi:10.1016/j.marenvres.2012.11.001.
Dailianis, S., Tsarpali, V., Melas, K., Karapanagioti, H. K., & Manariotis, I. D. (2014). Aqueous phenanthrene toxicity after high-frequency ultrasound degradation. Aquatic Toxicology, 147, 32–40.
de Lapuente, J., Lourenço, J., Mendo, S. A., Borràs, M., Martins, M. G., Costa, P. M., & Pacheco, M. (2015). The comet assay and its applications in the field of ecotoxicology: a mature tool that continues to expand its perspectives. Frontiers in Genetics, 6, 180.
Devin, S., Burgeot, T., Giambérini, L., Minguez, L., & Pain-Devin, S. (2014). The integrated biomarker response revisited: optimization to avoid misuse. Environmental Science and Pollution Research International, 21(4), 2448–54. doi:10.1007/s11356-013-2169-9.
Fernández, B., Campillo, J. A., Gómez, C. M., & Benedicto, J. (2012). Assessment of the mechanisms of detoxification of chemical compounds and antioxidant enzymes in the digestive gland of mussels, Mytilus galloprovincialis, from Mediterranean coastal sites. Chemosphere, 87, 1235–1245.
Frenzilli, G., Nigro, M., & Lyons, B. P. (2009). The comet assay for the evaluation of genotoxic impact in aquatic environments. Mutation Research Reviews in Mutation Research, 681, 80–92.
Frouin, H., Pellerin, J., Fournier, M., Pelletier, E., Richard, P., Pichaud, N., Rouleau, C., & Garnerot, F. (2007). Physiological effects of polycyclic aromatic hydrocarbons on soft-shell clam Mya arenaria. Aquatic Toxicology, 82, 120–134.
Gauthier, P. T., Norwood, W. P., Prepas, E. E., & Pyle, G. G. (2014). Metal-PAH mixtures in the aquatic environment: a review of co-toxic mechanisms leading to more-than-additive outcomes. Aquatic Toxicology, 154, 253–69.
Giannapas, M., Karnis, L., & Dailianis, S. (2012). Generation of free radicals in haemocytes of mussels after exposure to low molecular weight PAH components: immune activation, oxidative and genotoxic effects. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 155, 182–189.
Gowland, B. T. G., McIntosh, A. D., Davies, I. M., Moffat, C. F., & Webster, L. (2002). Implications of a field study regarding the relationship between polycyclic aromatic hydrocarbons and glutathione S-transferase activity in mussels. Marine Environmental Research, 54, 231–235.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases the first enzymatic step in mercapturic acid formation. The Journal of Biological Chemistry, 249(22), 7130–9.
Hannam, M. L., Bamber, S. D., Galloway, T. S., Moody, A. J., & Jones, M. B. (2010a). Effects of the model PAH phenanthrene on immune function and oxidative stress in the haemolymph of the temperate scallop Pecten maximus. Chemosphere, 78, 779–784.
Hannam, M. L., Bamber, S. D., Moody, A. J., Galloway, T. M., & Jones, M. B. (2010b). Immunotoxicity and oxidative stress in the arctic scallop Chlamys islandica: effects of acute oil exposure. Ecotoxicology and Environmental Safety, 73, 1440–1448.
IARC. (2010). Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures IARC monographs on the evaluation of carcinogenic risks to humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 92, 1–853.
Jarvis, I. W. H., Bergvall, C., Bottai, M., Westerholm, R., Stenius, U., & Dreij, K. (2013). Persistent activation of DNA damage signalling in response to complex mixtures of PAHs in air particulate matter. Toxicology and Applied Pharmacology, 266(3), 408–418.
Jifa, W., Yu, Z., Xiuxian, S., & You, W. (2006). Response of integrated biomarkers of fish (Lateolabrax japonicus) exposed to benzo[a]pyrene and sodium dodecylbenzene sulfonate. Ecotoxicology and Environmental Safety, 65, 230–236.
Kaloyianni, M., Dailianis, S., Chrisikopoulou, E., Zannou, A., Koutsogiannaki, S., Alamdari, D. H., Koliakos, G., & Dimitriadis, V. K. (2009). Oxidative effects of inorganic and organic contaminants on haemolymph of mussels. Comparative Biochemistry and Physiology - Part C, 149, 631–639.
Khan, M. A., Cheema, S. A., Tang, X., Hashmi, M. Z., Shen, C., Park, J., & Chen, Y. (2013). A battery of bioassays for the evaluation of phenanthrene biotoxicity in soil. Archives of Environmental Contamination and Toxicology, 65, 47–55.
Kim, W. K., Lee, S. K., & Jung, J. (2010). Integrated assessment of biomarker responses in common carp (Cyprinus carpio) exposed to perfluorinated organic compounds. Journal of Hazardous Materials, 180, 395–400.
Kopecka-Pilarczyk, J., & Correia, A. C. (2009). Biochemical response in gilthead seabream (Sparus aurata) to in vivo exposure to pyrene and fluorene. Journal of Experimental Marine Biology and Ecology, 372, 49–57.
Landrum, P. F., Dupuis, W. S., & Kukkonen, J. (1994). Toxicokinetics and toxicity of sediment associated pyrene and phenanthrene in Diporeia spp.: examination of equilibrium-partitioning theory and residue-based effects for assessing hazards. Environmental Toxicology and Chemistry, 13, 1769–1780.
Liu, C., Chang, V. W., Gin, K. Y., & Nguyen, V. T. (2014). Genotoxicity of perfluorinated chemicals (PFCs) to the green mussel (Perna viridis). The Science of the Total Environment, 487, 117–22. doi:10.1016/j.scitotenv.2014.04.017.
Livingstone, D. R. (2003). Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revista de Medicina Veterinaria, 154, 427–430.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). The original method. The Journal of Biological Chemistry, 193, 265.
Luchmann, K. H., Dafre, A. L., Trevisan, R., Craft, J. A., Meng, X., Mattos, J. J., Zacchi, F. L., Dorrington, T. S., Schroeder, D. C., & Bainy, A. C. D. (2014). A light in the darkness: new biotransformation genes, antioxidant parameters and tissue-specific responses in oysters exposed to phenanthrene. Aquatic Toxicology, 152, 324–334.
Machado, A. A., Hoff, M. L., Klein, R. D., Cordeiro, G. J., Lencina Avila, J. M., Costa, P. G., & Bianchini, A. (2014). Oxidative stress and DNA damage responses to phenanthrene exposure in the estuarine guppy Poecilia vivipara. Marine Environmental Research, 98, 96–105.
Martins, M., Costa, P. M., Ferreira, A. M., & Costa, M. H. (2013). Comparative DNA damage and oxidative effects of carcinogenic and non-carcinogenic sediment-bound PAHs in the gills of a bivalve. Aquatic Toxicology, 142–143, 85–95.
Martyniuk, C. J., Sanchez, B. C., Szabo, N. J., Denslow, N. D., & Sepúlveda, M. S. (2009). Aquatic contaminants alter genes involved in neurotransmitter synthesis and gonadotropin release in largemouth bass. Aquatic Toxicology, 95(1), 1–9.
Mashroofeh, A., Bakhtiari, A. R., & Pourkazemi, M. (2015). Distribution and composition pattern of polycyclic aromatic hydrocarbons in different tissues of sturgeons collected from Iranian coastline of the Caspian Sea. Chemosphere, 120, 575–83.
Mitchelmore, C. L., Birmelin, C., Chipman, J. K., & Livingstone, D. R. (1998). Evidence for cytochrome P-450 catalysis and free radical involvement in the production of DNA strand breaks by benzo[a]pyrene and nitroaromatics in mussel (Mytilus edulis L.) digestive gland cells. Aquatic Toxicology, 41, 193–212.
Namiesnik, J., Moncheva, S., Park, Y. S., Ham, K. S., Heo, B. G., Tashma, Z., Katrich, E., & Gorinstein, S. (2008). Concentration of bioactive compounds in mussels Mytilus galloprovincialis as an indicator of pollution. Chemosphere, 73(6), 938–944.
Niyogi, S., Biswas, S., Sarker, S., & Datta, A. G. (2001a). Antioxidant enzymes in brackish water oyster, Saccostrea cucullata as potential biomarkers of polyaromatic hydrocarbon pollution in Hooghly Estuary (India): seasonality and its consequences. The Science of the Total Environment, 281, 237–246.
Niyogi, S., Biswas, S., Sarker, S., & Datta, A. G. (2001b). Seasonal variation of antioxidant and biotransformation enzymes in barnacle, Balanus balanoides, and their relation with polyaromatic hydrocarbons. Marine Environmental Research, 52, 13–26.
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358. 177.
Oliveira, M., Pacheco, M., & Santos, M. A. (2007). Cytochrome P4501A, genotoxic and stress responses in golden grey mullet (Liza aurata) following short-term exposure to phenanthrene. Chemosphere, 66, 1284–1291.
Oliveira, M., Pacheco, M., & Santos, M. A. (2008). Organ specific antioxidant responses in golden grey mullet (Liza aurata) following a short-term exposure to phenanthrene. The Science of the Total Environment, 396, 70–78.
Olsvik, P. A., Nordtug, T., Altin, D., Lie, K. K., Overrein, I., & Hansen, B. H. (2010). Transcriptional effects on glutathione S-transferases in first feeding Atlantic cod (Gadus morhua) larvae exposed to crude oil. Chemosphere, 79, 905–913.
Oost, R., Beyer, J., & Vermeulen, N. P. E. (2003). Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology, 13, 57–149.
Pain-Devin, S., Cossu-Leguille, C., Geffard, A., Giambérini, L., Jouenne, T., et al. (2014). Towards a better understanding of biomarker response in field survey: a case study in eight populations of zebra mussels. Aquatic Toxicology, 155, 52–61.
Pan, L. Q., Ren, J., & Liu, J. (2006). Responses of antioxidant systems and LPO level tobenzo(a)pyrene and benzo(k)fluoranthene in the haemolymph of the scallop Chlamys ferrari. Environmental Pollution, 141, 443–451.
Pennec, G. L., & Pennec, M. L. (2003). Induction of glutathione-S-transferases in primary cultured digestive gland acini from the mollusk bivalve Pecten maximus (L.): application of a new cellular model in biomonitoring studies. Aquatic Toxicology, 64, 131–142.
Perez-Cadahia, B., Laffon, B., Pasaro, E., & Mendez, J. (2004). Evaluation of PAH bioaccumulation and DNA damage in mussels (Mytilus galloprovincialis) exposed to spilled prestige crude oil. Comparative Biochemistry and Physiology C, 138, 453–460.
Pichaud, N., Pellerin, J., Fournier, M., Gauthier-Clerc, S., Rioux, P., & Pelletier, E. (2008). Oxidative stress and immunologic responses following a dietary exposure to PAHs in Mya arenaria. Chemistry Central Journal, 2, 23.
Pisoni, M., Cogotzi, L., Frigeri, A., Corsi, I., Bonacci, S., Iacocca, A., Lancini, L., Mastrototaro, F., Focardi, S., & Svelto, M. (2004). DNA adducts, benzo(a)pyrene monooxygenase activity, and lysosomal membrane stability in Mytilus galloprovincialis from different areas in Taranto coastal waters (Italy). Environmental Research, 96(2), 63–175.
Regoli, F., & Giuliani, M. E. (2014). Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Marine Environmental Research, 93, 106–117.
Reitsema, T. J., & Spickett. (1999). Imposex in Morula Granulata as bioindicator of tributyltin (TBT) contamination in the Dampier Archipelago, Western Australia. Marine Pollution Bulletin, 39, 280–284.
Rostad, C. E., & Pereira, W. E. (1987). Creosote compounds in snails obtained from Pensacola Bay, Florida, near an onshore hazardous-waste site. Chemosphere, 16, 2397–2404.
Saint-Denis, M., Narbonne, J. F., Arnaud, C., Thybaud, E., & Ribera, D. (1999). Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil: effects of benzo(a)pyrene. Soil Biology and Biochemistry, 31, 1837–1846.
Sanders, M. (1994). Distribution of polycyclic aromatic hydrocarbons in oyster (Crassostrea virginica) and surface sediment from two estuaries in South Carolina. Archives of Environmental Contamination and Toxicology, 28, 397–405.
Sarkar, A., Gaitonde, D. C. S., Sarkar, A., Vashistha, D., D’Silva, C., & Dalal, S. G. (2008). Evaluation of impairment if DNA integrity in marine gastropods (Cronia contracta) as a biomarker of genotoxic contaminants in coastal water around Goa, West coast of India. Ecotoxicology and Environmental Safety, 71(2), 473–482.
Sarkar, A., Vashistha, D., Gupta,N., Malik,K., & Gaitonde, D.C.S. (2011). Measurement of DNA integrity in marine gastropods as biomarker of genotoxicity. In: Environmental pollution: ecological impacts. Bhattacharya, B., Ghosh, A., Majumdar, S.K. (Eds.) Health s and Management. Institute of Ecotoxicology and Environmental Sciences and Mudrakar, pp. 108–112.
Sarkar, A., Bhagat, J., & Sarkar, S. (2014). Evaluation of impairment of DNA in marine gastropod, Morula granulata as a biomarker of marine pollution. Ecotoxicology and Environmental Safety, 106, 253–261.
Sarkar, A., Bhagat, J., Ingole, B. S., Rao, D. P., & Markad, V. L. (2015). Genotoxicity of cadmium chloride in marine gastropod, Nerita chamaeleon using comet assay and alkaline unwinding assay. Environmental Toxicology, 30(2), 177–187.
Schirmer, K., Dixon, D. G., Greenberg, B. M., & Bols, N. C. (1998). Ability of 16 priority PAHs to be directly cytotoxic to a cell line from the rainbow trout gill. Toxicology, 127, 129–141.
Serafim, A., Company, R., Lopes, B., Fonseca, V. F., Franc¸, S., Vasconcelos, R. P., Bebianno, M. J., & Cabral, H. N. (2012). Application of an integrated biomarker response index (IBR) to assess temporal variation of environmental quality in two Portuguese aquatic systems. Ecological Indicators, 19, 215–225.
Silva, A. Z., Zanette, J., Ferreira, J. F., Guzenski, J., Marques, M. R. F., & Bainy, A. C. D. (2005). Effects of salinity on biomarker responses in Crassostrea rhizophorae (Mollusca, Bivalvia) exposed to diesel oil. Ecotoxicology and Environmental Safety, 62, 376–382.
Singh, N. P., McCoy, M. T., Tice, R. R., & Schneider, E. L. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research, 175, 184–191.
Sinha, A. K. (1972). Colorimetric assay of catalase. Analytical Biochemistry, 47, 389–394.
Souid, G., Souayed, N., Yaktiti, F., & Maaroufi, K. (2013). Effect of acute cadmium exposure on metal accumulation and oxidative stress biomarkers of Sparus aurata. Ecotoxicology and Environmental Safety, 89(1), 1–7.
Subba Rao, N. V., Dey, A., & Barua, S. (1992). Estuarine and marine molluscs fauna of West Bengal. Part 9 (State fauna series 3) (p. 129). Calcutta: Zoological Survey of India.
Sun, Y., Yu, H., Zhang, J., Yin, Y., Shi, H., & Wang, X. (2006). Bioaccumulation, depuration and oxidative stress in fish Carassius auratus under phenanthrene exposure. Chemosphere, 63(8), 1319–27.
Tian, S., Zhang, Y., Song, C., Zhu, X., & Xing, B. (2014). Titanium dioxide nanoparticles as carrier facilitate bioaccumulation of phenanthrene in marine bivalve, ark shell (Scapharca subcrenata). Environmental Pollution, 92, 59–64.
Tsangaris, C., Hatzianestis, I., Catsiki, V. A., Kormas, K. A., Strogyloudi, E., Neofitou, C., Andral, B., & Galgani, F. (2011). Active biomonitoring in Greek coastal waters: application of the integrated biomarker response index in relation to contaminant levels in caged mussels. The Science of the Total Environment, 412–413, 359–65.
Turja, R., Höher, N., Snoeijs, P., Baršienė, J., Butrimavičienė, L., Kuznetsova, T., Kholodkevich, S. V., Devier, M. H., Budzinski, H., & Lehtonen, K. K. (2014). A multibiomarker approach to the assessment of pollution impacts in two Baltic Sea coastal areas in Sweden using caged mussels (Mytilus trossulus). The Science of the Total Environment, 473–474, 398–409.
UNEP (1999). Inventory of information sources on chemicals persistent organic pollutants. 141pp
US EPA. (2009). United States Environmental Protection Agency Priority Pollutants. <http://www.epa.gov/waterscience/methods/pollutants.htm>.
Venier, P., & Canova, S. (1996). Formation of DNA adducts in the gill tissue of Mytilus galloprovincialis treated with benzo[a]pyrene. Aquatic Toxicology, 34, 119–133.
Woo, S., Kim, S., Yum, S., Yim, U. H., & Lee, T. K. (2006). Comet assay for the detection of genotoxicity in blood cells of flounder (Paralichthys olivaceus) exposed to sediments and polycyclic aromatic hydrocarbons. Marine Pollution Bulletin, 52(12), 768–1775.
Wood, A. W., Chang, R. L., Levin, W., Ryan, D. E., Thomas, P. E., Mah, H. D., Karle, J. M., Yagi, H., Jerina, D. M., & Conney, A. H. (1979). Mutagenicity and tumorigenicity of phenanthrene and chrysene epoxides and diol epoxides. Cancer Research, 39(10), 4069–77.
Wu, S., Wu, E., Qiu, L., Zhong, W., & Chen, J. (2011). Effects of phenanthrene on the mortality, growth, and anti-oxidant system of earthworms (Eisenia fetida) under laboratory conditions. Chemosphere, 83, 429–434.
Xu, W., Li, Y., Wu, Q., Wang, S., Zheng, H., & Liu, W. (2009). Effects of phenanthrene on hepatic enzymatic activities in tilapia (Oreochromis niloticus × O. aureus). Journal of Environmental Sciences, 21, 854–857.
Zhang, J., Wang, Z., Song, Z., Xie, Z., Li, L., & Song, L. (2012). Bioaccumulation of microcystins in two freshwater gastropods from a cyanobacteria-bloom plateau lake, Lake Dianchi. Environmental Pollution, 164, 227–234.
Zhang, H., Pan, L., & Tao, Y. (2014). Toxicity assessment of environmental pollutant phenanthrene in clam Venerupis philippinarum using oxidative stress biomarkers. Environmental Toxicology and Pharmacology, 3, 697–704.
Acknowledgments
The financial support by Department of Biotechnology, New Delhi, in the form of Senior Research Fellowship (SRF) to Jacky Bhagat is highly acknowledged. The authors also would like to thank the Director of CSIR-NIO for his hearted cooperation. This research was financially supported by Council of Scientific and Industrial Research (CSIR) through Project No. PSC0206. This is contribution no……….. of CSIR, NIO, Goa.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• An approach to evaluate genotoxicity of phenanthrene in marine gastropod was presented
• In vivo effects of phenanthrene in gastropod was evaluated using genotoxic and oxidative stress biomarker
• Multi-biomarker approach was assessed in marine gastropod
• Phenanthrene was found to be genotoxic to marine gastropod Morula granulata
• Positive correlations were observed in GST, LPO, and % DNA in tail in snails exposed to phenantherene
Rights and permissions
About this article
Cite this article
Bhagat, J., Sarkar, A. & Ingole, B.S. DNA Damage and Oxidative Stress in Marine Gastropod Morula granulata Exposed to Phenanthrene. Water Air Soil Pollut 227, 114 (2016). https://doi.org/10.1007/s11270-016-2815-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2815-1