Abstract
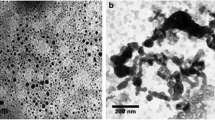
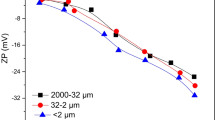
This study reports a method for preparation of pyrite nanoparticles and experimental results of reductive removal of perrhenate (a surrogate for radioactive pertechnetate) from water using the nanoparticles. The pyrite nanoparticles were prepared by ball milling in ethanol. Batch kinetic tests and column tests indicated that the pyrite nanoparticles were able to rapidly reduce and remove perrhenate at ambient temperatures. The pyrite nanoparticles were characterized using XRD and SEM. The effects of particle size and pH on the immobilization of perrhenate were studied in batch experiments with pyrite particles of different sizes (20 μm and 100 nm) and under different initial pH conditions (3.5, 6.6, and 12). To probe the potential of the nanoparticles for in situ immobilization of perrhenate in soil, column tests were carried out, where the pyrite nanoparticles were packed in the column to simulate a permeable reactive barrier. The column tests showed that the pyrite nanoparticles reduced the water leachable ReO4 − by 44 %. The results revealed great potential of pyrite nanoparticles for possible reductive immobilization of pertechnetate and other redox-sensitive metals and radionuclides in soil and groundwater.













Similar content being viewed by others
References
Aimoz, L., Curti, E., & Mader, U. (2011). Iodide interaction with natural pyrite. Journal Radioanalytical and Nuclear Chemistry, 288, 517–524.
Balfiz, P. (1998). Physic and chemical changes of sulphides during intensive grinding inorganic liquids. Powder Technology, 98(1), 74–78.
Cantrell, K. J., Kaplan, D. I., & Wietsma, T. W. (1995). Zero-valentiron for the in situ remediation of selected metals in groundwater. Journal of Hazardous Materials, 2(42), 201–212.
Chandra, A. P., & Gerson, A. R. (2010). The mechanisms of pyrite oxidation and leaching: a fundamental perspective. Surface Science Reports, 65, 293–315.
Dan, L. I., Zhoulan, Y. I. N., & Qiyuan, C. H. E. N. (2012). Interfacial and optical characteristics of pyrite ultrafine powder prepared using sodium salts as grinding additives. Journal of Central South University (Science and Technology), 43(5), 1645–1650.
Darab, J. G., Amonette, A. B., Burke, S. D., et al. (2007). Removal of pertechnetate from simulated nuclear waste streams using supported zerovalent iron. Chemistry of Materials, 19(23), 5703–5713.
Fan, Y., Tian-wei, Q., Qing-wei, D., et al. (2011). Kinetics of re deoxidized and fixed by nanoparticle-iron. Journal of Nuclear and Radiochemistry, 33(5), 280–284.
Farrell, J., Bostick, W., Jarabek, R., & Fiedor, J. (1999). Electrosorption and reduction of pertecnetate by anodically polarized magnetite. Environmental Science & Technology, 33, 1244–1249.
He, F., & Zhao, D. (2005). Preparation and characterization of a new class of starch-stabilized bimetallic nanoparticles for degradation of chlorinated hydrocarbons in water. Environmental Science and Technology, 39, 3314–3320.
He, F., Zhao, D., Liu, J., et al. (2007). Stabilization of Fe Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater. Industrial and Engineering Chemistry Research, 46, 29–34.
Hu, Q. H., Zavarin, M., & Rose, T. P. (2008). Effect of reducing groundwater on the retardation of redox-sensitive radionuclides. Geochemical Transactions, 9, 12. doi:10.1186/1467 -4866-9-12.
Huiping, H. U. (2003). Study on the rules for the changes of the structures and the properties of mechanically activated sulphide ores. Changsha: Central South University.
Jingping, Y., Yi, H., Jinfei, S., et al. (2008). Development of planetary mill and its energy saving mechanism. Journal of Southeast University (Natural Science Editon), 1, 27–31.
Kang, M., Chen, F., Wu, S., et al. (2011). Effect of pH on aqueous Se(IV) reduction by pyrite. Environmental Science & Technology, 45, 2704–2710.
Kaplan, D. I. (2003). Influence of surface charge of an Fe-oxide and an organic matter dominated soil on iodide and pertechnetate sorption. Journal Radiochimica Acta, 91, 173–178.
Lieser, K. H., & Bauscher, C. (1987). Technetium in the hydrosphere and in the geosphere I: chemistry of technetium and iron in natural waters and influence of the redox potential on the sorption of technetium. Radiochimica Acta, 42, 205–213.
Lloyd, J. R., Sole, V. A., & Lovley, D. R. (2000). Reducing Bacteria, 66(9), 2743–3749.
Poineau, F., & Mausolf. (2013). Technetium chemistry in the fuel cycle: combining basic and applied studies. Inorganic Chemistry, 52(7), 3573–3578.
Schwochau, K. (2000). Technetium: chemistry and radiopharmaceutical applications (pp. 43–44). Weinheim: Wiley-VCH.
Shi, J., & Lu, A. (2001). Experimental research on treatment of Cr(VI)–bearing wastewater by pyrite. Journal of Inner Mongolia University of Technology, 20(1), 6–9.
Speight, J. G. (2005). Lange’s handbook of chemistry. Sixteenth Edition: The McGraw Hill Companies, 395-399.
Um, W., Hyun-Shik, C., Jonathan, P., et al. (2011). Immobilization of 99-technetium (VII) by Fe(II)-goethite and limited reoxidation. Environtal Science and Technology, 45, 4904–4913.
Wang, & Zhang. (1997). Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environmental Science & Technology, 31, 2154–2156.
Wildung, R. E., McFadden, K. M., & Garland, T. R. (1979). Technetium sources and behavior in the environment. Journal of Environmental Quality, 8(2), 156–161.
Yongqiang, X. U. E. (2005). Effects of particle size on phase transitions and reactions of nanosystenms. Taiyuan: Taiyuan University of Technology.
Zhengguo, J. I. N., Ruisong, G. U. O., Chunsheng, S. H. I., et al. (2005). Fundamentals of material HYPERLINK "app:ds:science" \t "" science (pp. 147–149). Taijin: Tianjin University Press.
Acknowledgments
The authors would gratefully acknowledge the partial financial supports from the following sources: the National Natural Science Foundation of China (No. 41072265 and No. 41230638), International Cooperation Program of Shanxi Province (2010081021), Shanxi Scholarship Council of China (No. 2013-key 2), the Science and Technology Key Program of Shanxi Province (No. 20131101028), the Doctoral Program of Higher Education of Shanxi Province (No. 20101415110003), Students Innovative and Pioneering Program of Shanxi province (No. 120164075), and the Auburn University IGP program.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ding, Q., Ding, F., Qian, T. et al. Reductive Immobilization of Rhenium in Soil and Groundwater Using Pyrite Nanoparticles. Water Air Soil Pollut 226, 409 (2015). https://doi.org/10.1007/s11270-015-2588-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2588-y