Abstract
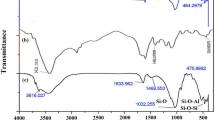
A bentonite-based hydrogel was chemically modified to prepare a new effective adsorbent for the removal of phosphate from aqueous solutions using batch equilibrium experiments at the laboratory scale. The efficiency of the phosphate adsorption by the modified adsorbents followed the order: Al-Fe-hydrogel > Al-hydrogel > Fe-hydrogel > Rewoquate surfactant-hydrogel ≅ Irasoft surfactant-hydrogel > raw hydrogel. The amount of Fe and Al, as determined in proportion to the cation exchange capacity (CEC) of the hydrogel, was the most important parameter for optimizing the modification process by pillaring solutions. The results showed that the phosphate adsorption was rapid and pH independent. The removal of phosphate reached up to 99 % at the optimized conditions. The adsorption data were well fitted by Langmuir and Freundlich models. According to the Langmuir model, the maximum adsorption capacity of the phosphate on the Fe-Al-hydrogel was 14.29 mg L−1. The removal of phosphate from an urban wastewater using the modified adsorbent was more than 99 %. The Fe-Al-hydrogel selectively adsorbed the phosphate from the solutions containing sulphate, bicarbonate, chloride, and nitrate. Based on the obtained results, the synthesized adsorbent could be used effectively to decontaminate the phosphate polluted water.






Similar content being viewed by others
References
Ahmed, E. M. (2013). Hydrogel: preparation, characterization, and applications. Journal of Advanced Research. doi:10.1016/j.jare.2013.07.006.
Bashour, I. I., & Sayegh, A. H. (2007). Methods of analysis for soils of arid and semi-arid regions (American University of Beirut, Lebanon). Rome: Food and Agriculture Organization of the United Nations (FAO).
Biswas, B. K., Inoue, K., Ghimire, K. N., Ohta, S., Harada, H., Ohto, K., et al. (2007). The adsorption of phosphate from an aquatic environment using metal-loaded orange waste. Journal of Colloid and Interface Science, 312(2), 214–223. doi:10.1016/j.jcis.2007.03.072.
Borgnino, L., Avena, M. J., & De Pauli, C. P. (2009). Synthesis and characterization of Fe(III)-montmorillonites for phosphate adsorption. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 341(1–3), 46–52. doi:10.1016/j.colsurfa.2009.03.037.
Borgnino, L., Giacomelli, C. E., Avena, M. J., & De Pauli, C. P. (2010). Phosphate adsorbed on Fe(III) modified montmorillonite: surface complexation studied by ATR-FTIR spectroscopy. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 353(2–3), 238–244. doi:10.1016/j.colsurfa.2009.11.022.
Bulut, Y., Akçay, G., Elma, D., & Serhatlı, I. E. (2009). Synthesis of clay-based superabsorbent composite and its sorption capability. Journal of Hazardous Materials, 171(1–3), 717–723.
Cheng, X., Huang, X., Wang, X., Zhao, B., Chen, A., & Sun, D. (2009). Phosphate adsorption from sewage sludge filtrate using zinc-aluminum layered double hydroxides. Journal of Hazardous Materials, 169(1–3), 958–964. doi:10.1016/j.jhazmat.2009.04.052.
Chitrakar, R., Tezuka, S., Sonoda, A., Sakane, K., Ooi, K., & Hirotsu, T. (2006). Selective adsorption of phosphate from seawater and wastewater by amorphous zirconium hydroxide. Journal of Colloid and Interface Science, 297(2), 426–433.
Chubar, N. I., Kanibolotskyy, V. A., Strelko, V. V., Gallios, G. G., Samanidou, V. F., Shaposhnikova, T. O., et al. (2005). Adsorption of phosphate ions on novel inorganic ion exchangers. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 255(1–3), 55–63. doi:10.1016/j.colsurfa.2004.12.015.
Clesceri, L. S., Greenberg, A. E., & Eaton, A. D. (1999). Standard Methods for the Examination of Water and Wastewater (20 ed.). Washington: American Public Health Association, American Water Works Association, Water Environment Federation.
Dable, P. J. M. R., Adjoumani, Y. J., Yao, B., & Ado, G. (2008). Wastewater dephosphorization using crude clays. Environmental Science Technology, 5(1), 35–42.
Dai, J., Yang, H., Yan, H., Shangguan, Y., Zheng, Q., & Cheng, R. (2011). Phosphate adsorption from aqueous solutions by disused adsorbents: Chitosan hydrogel beads after the removal of copper(II). Chemical Engineering Journal, 166(3), 970–977. doi:10.1016/j.cej.2010.11.085.
Darvishi, Z., & Morsali, A. (2010). Synthesis and characterization of Nano-bentonite by sonochemical method. Ultrasonics Sonochemistry, 18(1), 238–242.
De Vicente, I., Huang, P., Andersen, B., & Jensen, H. S. (2008). Phosphate adsorption by fresh and aged aluminum hydroxide. Consequense for lake restoration. Environmental Science Technology, 42(17), 6650–6655.
Dimirkou, A., Ioannou, A., & Doula, M. (2002). Preparation, characterization and sorption properties for phosphates of hematite, bentonite and bentonite-hematite systems. Advances in Colloid and Interface Science, 97(1–3), 37–60. doi:10.1016/S0001-8686(01)00046-X.
Evans, T. D., & Johnston, A. E. (2004). Phosphorus and crop nutrition: principles and practice. In E. Valsami-Jones (Ed.), Phosphorus in environmental technology (pp. 93–120). Cornwall, UK: IWA.
Fontes, M. P. F., & Weed, S. B. (1996). Phosphate adsorption by clays from Brazilian Oxisols: relationships with specific surface area and mineralogy. Geoderma, 72(1–2), 37–51.
Gan, F., Zhou, J., Wang, H., Du, C., & Chen, X. (2009). Removal of phosphate from aqueous solution by thermally treated natural palygorskite. Water Research, 43(11), 2907–2915. doi:10.1016/j.watres.2009.03.051.
González-Pradas, E., Villafranca-Sánchez, M., & Gallego-Campo, A. (1992). Effects of experimental variables on phosphate adsorption on bentonite. Journal of Chemical Technology and Biotechnology, 54(3), 291–295. doi:10.1002/jctb.280540313.
Gu, L., Xu, J., Lv, L., Liu, B., Zhang, H., Yu, X., et al. (2011). Dissolved organic nitrogen (DON) adsorption by using Al-pillared bentonite. Desalination, 269(1–3), 206–213. doi:10.1016/j.desal.2010.10.063.
Haghseresht, F., Wang, S., & Do, D. D. (2009). A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Applied Clay Science, 46(4), 369–375. doi:10.1016/j.clay.2009.09.009.
Hamdi, N., & Srasra, E. (2012). Removal of phosphate ions from aqueous solution using Tunisian clays minerals and synthetic zeolite. Journal of Environmental Sciences, 24(4), 617–623. doi:10.1016/S1001-0742(11)60791-2.
Jing, G., Wang, L., Yu, H., Amer, W. A., & Zhang, L. (2013). Recent progress on study of hybrid hydrogels for water treatment. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 416(0), 86–94. doi:10.1016/j.colsurfa.2012.09.043.
Kamiyango, M. W., Masamba, W. R. L., Sajidu, S. M. I., & Fabiano, E. (2009). Phosphate removal from aqueous solutions using kaolinite obtained from Linthipe, Malawi. Physics and Chemistry of the Earth, Parts A/B/C, 34(13–16), 850–856. doi:10.1016/j.pce.2009.07.012.
Kasama, T., Watanabe, Y., Yamada, H., & Murakami, T. (2004). Sorption of phosphates on Al-pillared smectites and mica at acidic to neutral pH. Applied Clay Science, 25(3–4), 167–177. doi:10.1016/j.clay.2003.09.005.
Khadhraoui, M., Watanabe, T., & Kuroda, M. (2002). The effect of the physical structure of a porous Ca-based sorbent on its phosphorus removal capacity. Water Research, 36, 3711–3718.
Kubilay, Ş., Gürkan, R., Savran, A., & Şahan, T. (2007). Removal of Cu(II), Zn(II) and Co(II) ions from aqueous solutions by adsorption onto natural bentonite. Adsorption, 13(1), 41–51. doi:10.1007/s10450-007-9003-y.
Li, Y., Liu, C., Luan, Z., Peng, X., Zhu, C., Chen, Z., et al. (2006). Phosphate removal from aqueous solutions using raw and activated red mud and fly ash. Journal of Hazardous Materials, 137(1), 374–383. doi:10.1016/j.jhazmat.2006.02.011.
Li, H., Ru, J., Yin, W., Liu, X., Wang, J., & Zhang, W. (2009). Removal of phosphate from polluted water by lanthanum doped vesuvianite. Journal of Hazardous Materials, 168(1), 326–330. doi:10.1016/j.jhazmat.2009.02.025.
Li, J., Li, Y., & Meng, Q. (2010). Removal of nitrate by zero-valent iron and pillared bentonite. Journal of Hazardous Materials, 174(1–3), 188–193. doi:10.1016/j.jhazmat.2009.09.035.
Liu, J., Zhou, Q., Chen, J., Zhang, L., & Chang, N. (2013). Phosphate adsorption on hydroxyl–iron–lanthanum doped activated carbon fiber. Chemical Engineering Journal, 215–216(0), 859–867. doi:10.1016/j.cej.2012.11.067.
Ma, J., & Zhu, L. (2006). Simultaneous sorption of phosphate and phenanthrene to inorgano-organo-bentonite from water. Journal of Hazardous Materials, 136(3), 982–988. doi:10.1016/j.jhazmat.2006.01.046.
Ma, J., Qi, J., Yao, C., Cui, B., Zhang, T., & Li, D. (2012). A novel bentonite-based adsorbent for anionic pollutant removal from water. Chemical Engineering Journal, 200–202(0), 97–103. doi:10.1016/j.cej.2012.06.014.
Morse, G. K., Brett, S. W., Guy, J. A., & Lester, J. N. (1998). Review: phosphorus removal and recovery technologies. The Science of The Total Environment, 212(1), 69–81. doi:10.1016/S0048-9697(97)00332-X.
Namasivayam, C., & Prathap, K. (2005). Recycling Fe(III)/Cr(III) hydroxide, an industrial solid waste for the removal of phosphate from water. Journal of Hazardous Materials, 123(1–3), 127–134. doi:10.1016/j.jhazmat.2005.03.037.
Namasivayam, C., & Sangeetha, D. (2004). Equilibrium and kinetic studies of adsorption of phosphate onto ZnCl2 activated coir pith carbon. Journal of Colloid and Interface Science, 280(2), 359–365. doi:10.1016/j.jcis.2004.08.015.
Oh, Y.-M., Hesterberg, D. L., & Nelson, P. V. (1999). Comparison of phosphate adsorption on clay minerals for soilless root media. Communications in Soil Science and Plant Analysis, 30(5&6), 747–756.
Peleka, E. N., & Deliyanni, E. A. (2009). Adsorptive removal of phosphates from aqueous solutions. Desalination, 245(1–3), 357–371. doi:10.1016/j.desal.2008.04.050.
Putra, E. K., Pranowo, R., Sunarso, J., Indraswati, N., & Ismadji, S. (2009). Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: mechanisms, isotherms and kinetics. Water Research, 43, 2419–2430.
Shirsath, S. R., Hage, A. P., Zhou, M., Sonawane, S. H., & Ashokkumar, M. (2011). Ultrasound assisted preparation of nanoclay Bentonite-FeCo nanocomposite hybrid hydrogel: a potential responsive sorbent for removal of organic pollutant from water. Desalination, 281(0), 429–437. doi:10.1016/j.desal.2011.08.031.
Tian, S., Jiang, P., Ning, P., & Su, Y. (2009). Enhanced adsorption removal of phosphate from water by mixed lanthanum/aluminum pillared montmorillonite. Chemical Engineering Journal, 151(1–3), 141–148. doi:10.1016/j.cej.2009.02.006.
Xiong, J. B., & Mahmood, Q. (2010). Adsorptive removal of phosphate from aqueous media by peat. Desalination, 259(1–3), 59–64. doi:10.1016/j.desal.2010.04.035.
Yan, L., Xu, Y., Yu, H. Q., Xin, X. D., Wei, Q., & Du, B. (2010). Adsorption of phosphate from aqueous solution by hydroxy-aluminum, hydroxy-iron and hydroxy-iron-aluminum pillared bentonites. Journal of Hazardous Materials, 179(1–3), 244–250. doi:10.1016/j.jhazmat.2010.02.086.
Yang, S., Zhao, Y., Chen, R., Feng, C., Zhang, Z., Lei, Z., et al. (2013). A novel tablet porous material developed as adsorbent for phosphate removal and recycling. Journal of Colloid and Interface Science, 396, 197–204. doi:10.1016/j.jcis.2012.12.077.
Yan-kui, T., Zhang-fa, T., Guang-tao, W., Zhong-min, L., & Da-wen, L. (2006). Removal of phosphate from aqueous solution with modified bentonite. The Chinese Journal of Process Engineering, 6(6), 197–200.
Yuan, P., He, H., Bergaya, F., Wu, D., Zhou, Q., & Zhu, J. (2006). Synthesis and characterization of delaminated iron-pillared clay with meso–microporous structure. Microporous and Mesoporous Materials, 88(1–3), 8–15. doi:10. 1016/j.micromeso.2005.08.022.
Zamparas, M., Drosos, M., Georgiou, Y., Deligiannakis, Y., & Zacharias, I. (2013). A novel bentonite-humic acid composite material Bephos™ for removal of phosphate and ammonium from eutrophic waters. Chemical Engineering Journal, 225, 43–51.
Zhang, G., Liu, H., Liu, R., & Qu, J. (2009). Removal of phosphate from water by a Fe-Mn binary oxide adsorbent. Journal of Colloid and Interface Science, 335(2), 168–174. doi:10.1016/j.jcis.2009.03.019.
Zhang, J., Shen, Z., Shan, W., Chen, Z., Mei, Z., Lei, Y., et al. (2010). Adsorption behavior of phosphate on Lanthanum(III) doped mesoporous silicates material. Journal of Environmental Sciences, 22(4), 507–511. doi:10.1016/S1001-0742(09)60141-8.
Zhao, D. L., Feng, S. J., Chen, C. L., Chen, S. H., Xu, D., & Wang, X. K. (2008). Adsorption of thorium(IV) on MX-80 bentonite: effect of pH, ionic strength and temperature. Applied Clay Science, 41, 17–23.
Zhao, Y., Wang, J., Luan, Z., Peng, X., Liang, Z., & Shi, L. (2009). Removal of phosphate from aqueous solution by red mud using a factorial design. Journal of Hazardous Materials, 165(1–3), 1193–1199. doi:10.1016/j.jhazmat.2008.10.114.
Zheng, H., Liu, D., Zheng, Y., Liang, S., & Liu, Z. (2009). Sorption isotherm and kinetic modeling of aniline on Cr-bentonite. Journal of Hazardous Materials, 167, 141–147.
Zhu, L., & Zhu, R. (2007). Simultaneous sorption of organic compounds and phosphate to inorganic–organic bentonites from water. Separation and Purification Technology, 54(1), 71–76. doi:10.1016/j.seppur.2006.08.009.
Zhu, R., Zhu, L., & Zhu, J. (2007). Simultaneous sorption of aqueous phenanthrene and phosphate onto bentonites modified with AlCl13 and CTMAB. Frontiers of Environmental Science & Engineering in China, 1(1), 79–82.
Zhu, M. X., Ding, K. Y., Xu, S. H., & Jiang, X. (2009a). Adsorption of phosphate on hydroxyaluminum- and hydroxyiron-montmorillonite complexes. Journal of Hazardous Materials, 165(1–3), 645–651. doi:10.1016/j.jhazmat.2008.10.035.
Zhu, R., Zhu, L., Zhu, J., Ge, F., & Wang, T. (2009b). Sorption of naphthalene and phosphate to the CTMAB-Al13 intercalated bentonites. Journal of Hazardous Materials, 168(2–3), 1590–1594. doi:10.1016/j.jhazmat.2009.03.057.
Acknowledgements
The authors wish to thank the Research Council of Isfahan University of Technology (IUT) for supporting this work and Prof. H. Shariatmadari from IUT for his scientific comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahni, S.Y., Mirghaffari, N., Rezaei, B. et al. Removal of Phosphate from Aqueous Solutions Using a New Modified Bentonite-Derived Hydrogel. Water Air Soil Pollut 225, 1916 (2014). https://doi.org/10.1007/s11270-014-1916-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-1916-y