Abstract
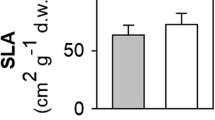
Plant physiological performance may be endangered in contaminated urban areas. Metal and polycyclic aromatic hydrocarbon (PAH) content in Quercus ilex leaves mainly reflects air pollution, being for these pollutants traceable the translocation from soil. In this work, in a field study, the responses at structural and functional level of Q. ilex leaves to metal and PAH pollution were assessed by an integrated approach. At this purpose, morphological (functional traits), biochemical (pigment content, antioxidant capacity, and PARP activity), and physiological (partitioning of absorbed light in the photosynthetic process) analyses were performed in leaves collected at urban parks and roadsides. Compared to the leaves of the urban parks, those sampled at the roadsides showed higher metal and PAH accumulation. The most contaminated leaves showed smaller leaf sizes and lower relative water and pigment content, as well as a lower photochemistry. In this circumstance, the excess of absorbed light at PSII was mainly dissipated via non-regulated than heat processes. On the basis of the biochemical analyses, the occurrence of DNA damages in contaminated Q. ilex leaves may be hypothesized.






Similar content being viewed by others
References
Agbaire, P. O., & Esiefarienrhe, E. (2009). Air pollution tolerance indices (APTI) of some plants around Otorogun gas plant in Delta state, Nigeria. Journal of Applied Sciences and Environmental Management, 13, 11–14.
Alfani, A., Baldantoni, D., Maisto, G., Bartoli, G., & Virzo De Santo, A. (2000). Temporal and spatial variation in C, N, S and trace element contents in the leaves of Quercus ilex within the urban area of Naples. Environmental Pollution, 109, 119–129.
Alfani, A., Maisto, G., Prati, M. V., & Baldantoni, D. (2001). Leaves of Quercus ilex L. as biomonitors of PAHs in the air of Naples (Italy). Atmospheric Environment, 35, 3553–3559.
Allen, R. (1995). Dissection of oxidative stress tolerance using transgenic plants. Plant Physiology, 107, 1049–1054.
Ambo-Rappe, R., Lajus, D. L., & Schreider, M. J. (2011). Heavy metal impact on growth and leaf asymmetry of seagrass, Halophila ovalis. Journal of Environmental Chemistry and Ecotoxicology, 3, 149–159.
Amor, E., Babiychuk, Y., Inzè, D., & Levine, A. (1998). The involvement of poly(ADP-ribose) polymerase in the oxidative stress responses in plants. FEBS Letters, 440, 1–7.
Arena, C., Vitale, L., & Virzo De Santo, A. (2005). Photosynthetic response of Quercus ilex L. plants grown on compost and exposed to increasing photon flux densities and elevated CO2. Photosynthetica, 43, 615–619.
Arena, C., Natale, E., Mistretta, C., Faraone Mennella, M. R., Virzo De Santo, A., & De Maio, A. (2011). Characterization and role of poly(ADP-ribosyl)ation in the Mediterranean species Cistus incanus L. under different temperature conditions. Plant Physiology and Biochemistry, 49, 435–440.
Arena, C., De Micco, V., De Maio, A., Mistretta, C., Aronne, G., & Vitale, L. (2013). Winter and summer leaves of Cistus incanus: differences in leaf morphofunctional traits, photosynthetic energy partitioning and poly(ADP ribose) polymerase activity. Botany, 91, 805–813. doi:10.1139/cjb-2013-0121.
Avila, A., & Rodrigo, A. (2004). Trace metal fluxes in bulk deposition, throughfall and stemflow at two evergreen oak stands in NE Spain subject to different exposure to the industrial environment. Atmospheric Environment, 38, 171–180.
Bargagli, R. (1998). Trace elements in terrestrial plants: an ecophysiological approach to biomonitoring and biorecovery. Berlin: Springer-Verlag.
Belpomme, D., Irigaray, P., Hardell, L., Clapp, R., Montagniere, L., Epstein, S., et al. (2007). The multitude and diversity of environmental carcinogens. Environmental Research, 105, 414–429.
Benavides, M. P., Gallego, S. M., & Tomaro, M. L. (2005). Cadmium toxicity in plants. Brazilian Journal of Plant Physiology, 17, 21–34.
Bilger, W., & Björkman, O. (1990). Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynthesis Research, 25, 173–185.
Briggs, A. G., & Bent, A. F. (2011). Poly(ADP-ribosyl)ation in plants. Trends in Plant Science, 16, 372–380.
Caporn, S. J. M. (2013). Ecophysiological responses of plants to air pollution. In: eLS. Wiley, Chichester. http://www.els.net/. doi:10.1002/9780470015902.a0003206.pub2.
Cornelissen, J. H. C., Lavorel, S., Garnier, E., Dìaz, S., Buchmann, N., Gurvich, D. E., et al. (2003). Handbook of protocols for standardized and easy measurements of plant functional traits worldwide. Australian Journal of Botany, 51, 335–380.
De Block, M., Verduyn, C., De Brouwer, D., & Cornelissen, M. (2005). Poly(ADP-ribose) polymerase in plants affects energy homeostasis, cell death and stress tolerance. Plant Journal, 41, 95–106.
De Nicola, F., Maisto, G., & Alfani, A. (2003). Assessment of nutritional status and trace element contamination of holm oak woodlands through analyses of leaves and surrounding soils. The Science of the Total Environment, 311, 191–203.
De Nicola, F., Maisto, G., Prati, M. V., & Alfani, A. (2005). Temporal variations in PAH concentrations in Quercus ilex L. (holm oak) leaves in an urban area. Chemosphere, 61, 432–440.
De Nicola, F., Maisto, G., Prati, M. V., & Alfani, A. (2008). Leaf accumulation of trace elements and polycyclic aromatic hydrocarbons (PAHs) in Quercus ilex L. Environmental Pollution, 153, 376–383.
De Nicola, F., Alfani, A., & D'Ambrosio, N. (2011a). Impact of the Mediterranean urban environment on photosynthetic efficiency of Quercus ilex leaves. Water, Air, & Soil Pollution, 220, 151–160.
De Nicola, F., Lancellotti, C., Prati, M. V., Maisto, G., & Alfani, A. (2011b). Biomonitoring of PAHs by using Quercus ilex leaves: source diagnostic and toxicity assessment. Atmospheric Environment, 45, 1428–1433.
Di Gennaro, A., & Terribile, F. (1999). I suoli della provincia di Napoli. Firenze: Selca.
Foyer, C. H., Lelandais, M., & Kunert, K. J. (1994). Photooxidative stress in plants. Physiologia Plantarum, 92, 696–717.
Gratão, P. L., Monteiro, C. C., Carvalho, R. F., Tezotto, T., Piotto, F. A., Peres, L. E. P., et al. (2012). Biochemical dissection of diageotropica and Never ripe tomato mutants to Cd-stressful conditions. Plant Physiology and Biochemistry, 56, 79–96.
Heyno, E., Klose, C., & Krieger-Liszkay, A. (2008). Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytology, 179, 687–699.
Hirotsu, N., Makino, A., Ushio, A., & Mae, T. (2004). Changes in thermal dissipation and the electron flow in the water–water cycle in rice grown under conditions of physiologically low temperature. Plant & Cell Physiology, 5, 635–644.
Horváth, G., Droppa, M., Oravecz, Á., Raskin, V. I., & Marder, J. B. (1996). Formation of the photosynthetic apparatus during greening of cadmium-poisoned barley leaves. Planta, 199, 238–243.
Howsam, M., & Jones, K. C. (1998). Sources of PAHs in the environment. The handbook of environmental chemistry. Berlin: Springer.
IARC. (1987). Overall evaluations of carcinogenicity: an updating of IARC monographs. Monographs on the evaluation of the carcinogenic risk of chemicals to humans, Vol. 1–2, Suppl. 7. Lyon: International Agency for Research on Cancer.
Jaspers, P., Overmyer, K., Wrzaczek, M., Vainonen, J. P., Blomster, T., Salojärvi, J., Reddy, R. A., & Kangasjärvi, J. (2010). The RST and PARP-like domain containing SRO protein family: analysis of protein structure, function and conservation in land plants. BMC Genomics, 11, 170. http://www.biomedcentral.com/1471-2164/11/170
Karuppanapandian, T., Sinha, P. B., Premkumar, G., & Manoharan, K. (2006). Chromium toxicity: correlated with increased in degradation of photosynthetic pigments and total soluble protein and increased peroxidase activity in green gram (Vigna radiata L.) seedlings. Journal of the Swamy Botanical Club, 23, 117–122.
Kramer, D. M., Johnson, G., Kiirats, O., & Edwards, G. E. (2004). New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynthesis Research, 79, 209–218.
Kumar, S. P., Mozhi Varman, P. A., & Kumari, B. D. R. (2011). Identification of differentially expressed proteins in response to Pb stress in Catharanthus roseus. African Journal of Environmental Science and Technology, 5, 689–699.
Lamb, R. S., Citarelli, M., & Teotia, S. (2012). Function of poly (ADP-ribose) polymerase superfamily in plants. Cellular and Molecular Life Science, 69, 175–189.
Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods in Enzymology, 148, 350–382.
Lichtenthaler, H. K., & Wellburn, A. R. (1983). Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions, 11, 591–592.
Maisto, G., Alfani, A., Baldantoni, D., De Marco, A., & Virzo De Santo, A. (2004). Trace metals in the soil and in Q. ilex L. leaves at anthropic and remote sites of the Campania Region of Italy. Geoderma, 122, 269–279.
Martìnez-Garza, C., Peña, V., Ricker, M., Campos, A., & Howe, H. F. (2005). Restoring tropical biodiversity: leaf traits predict growth and survival of late-successional trees in early-successional environments. Forest Ecology and Management, 217, 365–379.
Meot-Duros, L., & Magné, C. (2009). Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiology and Biochemistry, 47, 37–41.
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7, 405–410.
Osório, M. L., Osório, J., Vieira, A. C., Gonçalves, S., & Romano, A. (2011). Influence of enhanced temperature on photosynthesis, photooxidative damage, and antioxidant strategies in Ceratonia siliqua L. seedlings subjected to water deficit and rewatering. Photosynthetica, 49, 3–12.
Oxborough, K., & Baker, N. R. (1997). Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—calculation of qp and Fv′/Fm′ without measuring Fo′. Photosynthesis Research, 54, 135–142.
Peñuelas, J., Munné-Bosch, S., Llusià, J., & Filella, I. (2004). Leaf reflectance and photo- and antioxidant protection in field-grown summer-stressed Phillyrea angustifolia. Optical signals of oxidative stress. New Phytologist, 162, 115–124.
Polle, A. (2001). Dissecting the superoxide dismutase–ascorbate–glutathione-pathway in chloroplasts by metabolic modelling. Computer simulations as a step towards flux analysis. Plant Physiology, 126, 1445–1462.
Prieto, P., Pineda, M., & Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry, 269, 337–341.
Rizhsky, L., Liang, H., & Mittler, R. (2002). The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiology, 130, 1143–1151.
Rossini Oliva, S., & Espinosa, A. J. F. (2007). Monitoring of heavy metals in topsoils, atmospheric particles and plant leaves to identify possible contamination sources. Microchemical Journal, 86, 131–139.
Santorufo, L., Van Gestel, C. A. M., Rocco, A., & Maisto, G. (2012). Soil invertebrates as bioindicators of urban soil quality. Environmental Pollution, 161, 57–63.
Seyyednejad, S. M., Niknejad, M., & Koochak, H. (2011). A review of some different effects of air pollution on plants. Journal of Environmental Science, 10, 302–309.
Shakya, K., Chettri, M. K., & Sawidis, T. (2008). Impact of heavy metals (copper, zinc and lead) on the chlorophyll content of some mosses. Archives of Environmental Contamination and Toxicology, 54, 412–421.
Tian, R., Zhang, G. Y., Yan, C. H., & Dai, Y. R. (2000). Involvement of poly(ADP-ribose) polymerase and activation of caspase-3-like protease in heat shock-induced apoptosis in tobacco suspension cells. FEBS Letters, 474, 11–15.
Tiwari, B. S., Belenghi, B., & Levine, A. (2002). Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiology, 128, 1271–1281.
Tiwari, S., Agrawal, M., & Marshall, F. M. (2006). Evaluation of ambient air pollution impact on carrot plants at a sub urban site using open top chambers. Environmental Monitoring and Assessment, 119, 15–30.
Wang, L. J., Loescher, W., Duan, W., Li, W. D., Yang, S. H., & Li, S. H. (2009). Heat acclimation induced acquired heat tolerance and cross adaptation in different grape cultivars: relationships to photosynthetic energy partitioning. Functional Plant Biology, 36, 516–526.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arena, C., De Maio, A., De Nicola, F. et al. Assessment of Eco-Physiological Performance of Quercus ilex L. Leaves in Urban Area by an Integrated Approach. Water Air Soil Pollut 225, 1824 (2014). https://doi.org/10.1007/s11270-013-1824-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1824-6