Abstract
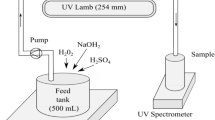
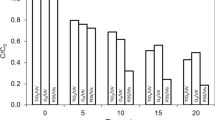
Large quantities of pollutants such as phthalocyanine which are difficult to degrade by conventional techniques are discharged by the textile industry. Advanced oxidation processes have been shown to be capable of degrading organic compounds and removing colour from the industrial wastewater. In this research, the hydrogen peroxide (H2O2)/UV process under different operative variables has been checked using a photoreactor lab plant to analyse its behaviour in the removal of colour and chemical oxygen demand of synthetic textile wastewater with a pigment named phthalo blue 36:3 (C.I. PB15:3). Different pH and H2O2 concentrations were tested to find the better conditions for the UV/H2O2 process suitable for this kind of pollution; this was carried out as an initial study of the operative variables for the scale-up of this technology. The research has shown that with pH 7 and 5 g/L of H2O2, this process can get an organic matter removal higher than 89 % and a colour removal near 70 %. Different kinetic models of organic matter removal have been checked to analyse and predict the influence of time on the process to model similar conditions of pollution. The high correlation between empirical and theoretical data model was pseudofirst order (R 2 = 0.989 ± 0.007).




Similar content being viewed by others
References
Alinsafi, A., Evenou, F., Abdulkarim, E. M., Pons, M. N., Zahraa, O., Benhammou, A., et al. (2007). Treatment of textile industry wastewater by supported photocatalysis. Dyes and Pigments, 74, 439–445.
Alnaizy, R., & Akgerman, A. (2000). Advanced oxidation of phenolic compounds. Advances in Environmental Research, 4, 233–244.
Antoniadis, A., Takavakoglou, V., Zalidis, G., Darakas, E., & Poulios, I. (2010). Municipal wastewater treatment by sequential combination of photocatalytic oxidation with constructed wetlands. Catalysis Today, 151, 114–118.
APHA, AWWA, WEF. (2005). Standard methods for the examination of water and wastewater (20th ed.). Washington, DC: American Public Health Association.
Alaton, I. A., Akmehmet Balcioglu, I., & Bahnemann, D. F. (2002). Advanced oxidation of a reactive dyebath effluent: comparison of O3, H2O2/UV-C and TiO2/UV-A processes. Water Research, 36, 1143–1154.
Bali, U., Çatalkayab, E., & Sengül, F. (2004). Photodegradation of Reactive Black 5, Direct Red 28 and Direct Yellow 12 using UV, UV/H2O2 and UV/H2O2/Fe2+: a comparative study. Journal of Hazardous Materials, B114, 159–166.
Calero, M., Blázquez, G., & Martín-Lara, M. A. (2011). Kinetic modelling of the biosorption of lead (II) from aqueous solutions by solid waste resulting from the olive oil production. Chemical & Engineering Data, 56, 3053–3060.
Crittenden, J. C., Hu, S., Hand, D. W., & Green, S. A. (1999). A kinetic model for H2O2/UV process in a completely mixed batch reactor. Water Research, 33(10), 2315–2328.
Elyasi, S., & Taghipour, F. (2010). Simulation of UV photoreactor for degradation of chemical contaminants: model development and evaluation. Environmental Science and Technology, 44, 2056–2063.
Fatta-Kassinos, D., Vasquez, M. I., & Kümmerer, K. (2011). Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes—degradation, elucidation of by products and assessment of their biological potency. Chemosphere, 85, 693–709.
Forgacs, E., Cserháti, T., & Oros, G. (2004). Removal of synthetic dyes from wastewaters: a review. Environment International, 30(7), 953–971.
Fu, L.-y., Wen, X.-h., Xu, L.-j., & Qian, Y. (2002). Removal of a copper-phthalocyanine dye from wastewater by acclimated sludge under anaerobic or aerobic conditions. Process Biochemistry, 37, 1151–1156.
Garcia-Ochoa, F., & Gomez, E. (2009). Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnology Advances, 27, 153–176.
Ghasemi, M., Wan Daud, W. R., Rahimnejad, M., Rezayi, M., Fatemi, A., Jafari, Y., Somalu, M. R., & Manzour, A. (2013). Copper-phthalocyanine and nickel nanoparticles as novel cathode catalysts in microbial fuel cells. International Journal of Hydrogen Energy, 38(22), 9533–9540.
Gil Rodríguez, M. (1998). Demanda bioquímica de oxígeno de efluentes con productos xenobióticos. Ingeniería del Agua, 5(4), 47–54.
Glaze, W. H., Kang, J. W., & Chapin, D. H. (1987). The chemistry of water treatment processes involving ozone, hydrogen peroxide and UV-radiation. Ozone: Science & Engineering, 9, 335–352.
Ho, Y. S., & McKay, G. (1998). Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70, 115–124.
Ho, Y. S., Ng, J. C. Y., & Mckay, G. (2000). Kinetics of pollutant sorption by biosorbents: review. Separation and Purification Reviews, 29(2), 189–232.
Jancûla, D., & Maršalek, B. (2011). The toxicity of phthalocyanines to the aquatic plant Lemna minor (duckweed)—testing of 31 compounds. Chemosphere, 88, 962–965.
Ju, L.-K., & Chase, G. G. (1992). Improved scale-up strategies of bioreactors. Bioprocess Engineering, 8, 49–53.
Junker, B. H. (2004). Scale-up methodologies for Escherichia coli and yeast fermentation processes. Journal of Bioscience and Bioengineering, 97(6), 347–364.
Kalsoom, U., Ashraf, S. S., Meetani, M. A., Rauf, M. A., & Bhatti, H. N. (2012). Degradation and kinetics of H2O2 assisted photochemical oxidation of Remazol Turquoise Blue. Chemical Engineering Journal, 200–202, 373–379.
Kralik, P., Kusic, H., Koprivanac, N., & Bozic, A. L. (2010). Degradation of chlorinated hydrocarbons by UV/H2O2: the application of experimental design and kinetic modelling approach. Chemical Engineering Journal, 158, 154–166.
Kumar Verma, A., Roshan Dash, R., & Bhunia, P. (2012). A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. Journal of Environmental Management, 93(1), 154–168.
Kusic, H., Koprivanac, N., Horvat, S., Bakija, S., & Bozi, A. L. (2009). Modelling dye degradation kinetic using dark- and photo-Fenton type processes. Chemical Engineering Journal, 155, 144–154.
Lan, B. Y., Nigmatullin, R., & Li Puma, G. (2008). Ozonation kinetics of cork-processing water in a bubble column reactor. Water Research, 42, 2473–2482.
Lucas, M. S., Mosteo, R., Maldonado, M. I., Malato, S., & Peres, J. A. (2009a). Solar photochemical treatment of winery wastewater in a CPC reactor. Journal of Agricultural and Food Chemistry, 57, 11242–11248.
Lucas, M. S., Peres, J. A., Lan, B. Y., & Li Puma, G. (2009b). Ozonation kinetics of winery wastewater in a pilot-scale bubble column reactor. Water Research, 43, 1523–1532.
McMullan, G., Meehan, G., Conneely, A., Kirby, N., Robinson, T., Nigam, P., et al. (2001). Microbial decolourisation and degradation of textile dyes. Applied Microbiology and Biotechnology, 56, 81–87.
Poyatos, J. M., Muñio, M. M., Almecija, M. C., Torres, J. C., Hontoria, E., & Osorio, F. (2010). Advanced oxidation processes for wastewater treatment: state of the art. Water, Air, and Soil Pollution, 205, 187–204.
Rajeshwar, K., Osugi, M. E., Chanmanee, W., Chenthamarakshan, C. R., Zanoni, M. V. B., Kajitvichyanukul, P., et al. (2008). Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. Journal of Photochemistry and Photobiology C Photochemistry, 9, 171–192.
Rauf, M. A., & Ashraf, S. S. (2012). Survey of recent trends in biochemically assisted degradation of dyes. Chemical Engineering Journal, 209, 520–530.
Rismayani, S., Fukushima, M., Ichikawa, H., & Tatsumi, K. (2004). Decolourisation of orange II by catalytic oxidation using iron (III) phthalocyanine-tetrasulphonic acid. Journal of Hazardous Materials, B114, 175–181.
Rizzo, L. (2011). Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Research, 45, 4311–4340.
Santos, A., Ystos, P., Rodríguez, S., & Romero, A. (2010). Mineralisation lumping kinetic model for abatement of organic pollutants using Fenton’s reagent. Catalysis Today, 151, 89–93.
Sarria, V., Parra, S., Adler, N., Péringer, P., Benitez, N., & Pulgarin, C. (2002). Recent developments in the coupling of photoassisted and aerobic biological processes for the treatment of biorecalcitrant compounds. Catalysis Today, 76, 301–315.
Schrank, S. G., Ribeiro dos Santos, J. N., Santos Souza, D., & Santos Souza, E. E. (2007). Decolourisation effects of Vat Green 01 textile dye and textile wastewater using H2O2/UV process. Journal of Photochemistry and Photobiology, A: Chemistry, 186, 125–129.
Sena, S., & Demirer, G. N. (2003). Anaerobic treatment of real textile wastewater with a fluidized bed reactor. Water Research, 37, 1868–1878.
Shu, H.-Y., & Chang, M.-C. (2005). Decolourisation and mineralisation of a phthalocyanine dye C.I. Direct Blue 199 using UV/H2O2 process. Journal of Hazardous Materials, B125, 96–101.
Silva, M. C., Duarte Corrêa, A., Sousa Pessoa Amorim, M. T., Parpotc, P., Arriel Torres, J., & Batista Chagas, P. M. (2012). Decolourisation of the phthalocyanine dye reactive blue 21 by turnip peroxidase and assessment of its oxidation products. Journal of Molecular Catalysis B: Enzymatic, 77, 9–14.
Wenzel, A., Gahr, A., & Niessner, R. (1999). TOC-removal and degradation of pollutants in leachate using a thin-film photoreactor. Water Research, 33(4), 937–946.
Yonar, T., Kestioglu, K., & Azbar, N. (2006). Treatability studies on domestic wastewater using UV/H2O2 process. Applied Catalysis B: Environmental, 67, 223–228.
Acknowledgments
This research was supported by University of Granada under project reference no. CTM2009-11929-C02-02 by a personal grant to C. Lopez-Lopez.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopez-Lopez, C., Martín-Pascual, J., Martínez-Toledo, M.V. et al. Effect of the Operative Variables on the Treatment of Wastewater Polluted with Phthalo Blue by H2O2/UV Process. Water Air Soil Pollut 224, 1725 (2013). https://doi.org/10.1007/s11270-013-1725-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1725-8