Abstract
Photocatalytic activity in titanium dioxide (TiO2) has been extensively studied because of its potential use in sterilization, sanitation, and remediation applications. The aim of the study reported here was to assess the feasibility of “fixed” TiO2 as the photocatalyst for inactivating pathogenic bacteria selected, Staphylococcus aureus and Escherichia coli, from a water stream. The investigation was undergone in a properly designed laboratory-scale evaluation. Using the system reported here, we obtained an effective bactericidal capability for E. coli and S. aureus with 90.0% and 98.0% after 30 and 10 min ultraviolet-A light irradiation with fixed TiO2, respectively. Parameters such as the various initial bacteria concentration, TiO2 concentrations, interruption of illumination, turbidity, and coexisted organic matters were examined to identify the removal efficiency in the photocatalytic reaction. Results indicated the negative effect by high bacteria concentration, coexisted organic matters, and turbidity on inactivation of bacteria, and positive effect on disinfection was associated with higher TiO2 concentration. Furthermore, our results indicated that under the same experimental conditions, the removal efficiency of the system in synthetic water was performed better than that of crude water. This inferior removal capability in crude water is mainly caused by the negative effect from the unknown coexisted factors.
Similar content being viewed by others
1 Introduction
Microorganisms are the cause of many infectious diseases that are still the leading cause of death to the host. Prevention recognizes the control of infectious diseases from microorganisms is very important in healthcare, food, and drinking water. However, in developing countries with a deteriorating environment, the demand for clean drinking water supply is growing rapidly in recent years (Gelover et al. 2006). The research provided persuasive evidence that certain microorganisms were responsible for human illness. Microorganisms are the cause of the majority of food-borne illness. Food-borne infections result when a person eats food that contains pathogens, which then produce illness-causing toxins in the intestines. Once microorganisms are discharged into the environment, they are sensitive to the status of the environment. Temperature, ultraviolet (UV) radiation, or other environmental factors may destroy the microorganisms present in polluted water (Hijnen et al. 2006). Because of the widespread use of antibiotics and the emergence of more resistant and virulent strains of microorganisms, there is an immediate need to develop alternative sterilization technologies, and the control of environmental factors that affect the inactivation of bacteria is critical.
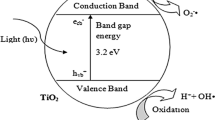
Direct UV irradiation is an attractive disinfection method for inactivating bacteria. However, exposure to UV radiation may result in acute and chronic health effects on the skin, eyes, and immune system (Kuhn et al. 2003). The previous work on photocatalytic disinfection has recently been reviewed. The titanium dioxide (TiO2) photocatalytic process is a conceptually simple and promising technology, providing an indirect radiation and high rate of sterilization at room temperature (Kim et al. 2003). Also, TiO2 acts as a photocatalyst which has strong oxidizing and decomposing effects when activated by UV light (Rincón and Pulgarin 2003). When TiO2 is irradiated with UV light, free hydroxyl ·OH radicals are generated which exhibit strong bactericidal activity (Rincón and Pulgarin 2003). Unfortunately, TiO2 suspended in crude water exhibited considerably less feasibility in real application (Cushnie et al. 2009). Conversely, methods of coating the TiO2 onto the material in the crude water demonstrated better performance in antibacterial activities. Variables that affect the experimental results include initial microorganism concentration, photocatalyst concentration, UV light intensity, temperature, pH value, and microorganism type (Hijnen et al. 2006; McCullagh et al. 2007).
The aim of the present study was to use Staphylococcus aureus and Escherichia coli as biological indicators of removal efficiency in tap water infection system with TiO2 coating and then to evaluate the photocatalytic performance on the sterilization process that would be influenced by various physical parameters to inactivate the bacteria. Furthermore, the parameters such as interruption of illumination, the amount of TiO2, initial bacteria concentration, turbidity, and coexisting organic matters have been evaluated. Also, using the system reported here, we evaluated and compared the durability of disinfection after direct photolysis and photocatalysis, respectively. Moreover, we demonstrate, for the first time, the efficiency of bacteria removal using fixed TiO2 in comparing synthetic water and crude water.
2 Experimental
2.1 Bacterial Strain and Growth Media
Gram-positive S. aureus ATCC 6538 and Gram-negative E. coli ATCC 10536 (Bioresource Collection and Research Center; HsinChu, Taiwan) were subcultured and maintained on nutrient agar. To provide the bacterial cultures, the isolated colonies with similar morphological type were removed from the plates, then inoculated on 100 mL nutrient broth and incubated at 37°C in an orbital incubator set at 200 rpm. At an exponential growth phase of OD600 0.8, bacterial cells were collected by centrifugation at 5,000×g for 10 min at 4°C, and the bacterial pellet was washed three times with deionized water. Eventually, the bacterial pellet was resuspended in deionized water, thereby providing a formulated concentration of cell suspensions used in this research.
2.2 Photocatalytic Reaction in Petri Dish Experiment
The TiO2 film was prepared via a modified procedure of Ao et al. (2007). Fifteen milliliters titanium isopropoxide and 1 ml nitric acid were orderly added into a glass container containing 100 ml deionized water and then refluxed in a stirring flask at 70°C for 2 days to produce sol–gel solution. At this time, the solution was poured onto the inner surface of a clean (DI 87 mm) glass Petri dish. To obtain the photocatalytic synthetic film sample, the TiO2-coated dish was then heated in an oven for 24 h at 90°C. The photocatalytic oxidation experiment was prepared in a procedure by transferring bacterial cells (approximately 107 CFU/ml) into the glass Petri dish (bottom coated with TiO2) containing 15 ml of sterilized deionized water and then exposed vertically to UV radiation for a span (UV lamp; TL 20 W/05, Philips. Co, Holland). In accordance with the results presented from the early experiment, the 366 nm (UVA) performs much better than the 254 nm (UVC) in bacteria inactivation; thereby, the peak wavelength of UV lamps was set at 366 nm (UVA), and the light intensity was monitored by a quantum photo/radio meter (Delta ohm, HG 9021, Italy), with a distance of 15 cm to the surface of Petri dish that has been confirmed by the early experiment as the same effect with distance from 6 to 15 cm. The light intensity at such distance was calculated to be 3.4 × 10−5 Einsteins s−1. In our study, photocatalytic, adsorptive, UV, and control methods were used to determine the possible bacteria inactivation mechanisms involved in TiO2 removal of bacteria in a similar water sample. Using the system reported here, photocatalytic procedure was deliberately followed; adsorptive results were collected from the identical surrounding condition without exposure to UV; the UV irradiation was practiced on the similar photocatalytic environment without TiO2 coating; results of the control sample without both TiO2 coating and UV irradiation were used to determine the removal capability of the test sample. To understand the effect of different parameters on removal efficiency, we have investigated critical parameters, such as TiO2 amount, initial bacterial concentration, turbidity, and coexisting organic matters. Among these parameters, suspended bentonite and kaolin produced different turbidity during the process which was measured by Hach model 2100A turbidimeter, and samples were taken for each of the following times (5 or 10 min, up to 1.5 h). All experiments were done in triplicates.
2.3 Comparison of Synthetic Water and Crude Water
A comparison of the data presented in synthetic and crude water was designed to evaluate the bacterial removal efficiency of the system with TiO2 coating. Synthesized water was made by 15 mL iso-osmotic 0.9% (w/v) NaCl solution, with the concentration of E. coli or/and S. aureus of 107 CFU/mL, and crude water was collected from Syh-Fen Stream (Nangang, Taipei, Taiwan) and pretreated in a filter to reduce the concentration of suspended solids and turbidity then sterilized by autoclaving. At this time, 107 CFU/mL of E. coli and S. aureus were placed into the TiO2-coated glass dishes. Thereafter, the dishes containing the test bacteria were exposed continuously to UV radiation for up to 30 min. The UV was then switched off and allowed the test sample to be place on the unchanged environment for 24 h before inspected in the experiments. To evaluate the changes in the bacteria amounts in the test sample, the control experiment was conducted on the identical experimental condition without UV irradiation, thereby providing the change of bacteria count shown in Section 2.4.
2.4 Bacterial Count
Bacterial counts in bedding profoundly affect the microbial populations on test sample. In this study, the spread plate method was employed to estimate total numbers of colony-forming units (CFU) in samples using nutrient agar as medium grown at 37°C for 18 h. Serial dilutions are prepared so that following incubation, a certain volume of suspension withdrawn from each sample was diluted to 1/10, 1/100, and 1/1,000 of its initial concentration with deionized water. At this time, the nutrient agar plate is inoculated from the dilution (which has been thoroughly mixed) or directly from the sample using a 0.1-mL inoculum, if low counts are expected. To obtain the bacterial count, the inoculum is then uniformly spread over the surface, and these samples were cultured for 18 h.
3 Results and Discussion
3.1 Effect of the UV Light in the Presence or Absence of TiO2
The sunlight is able to inactivate bacteria due to the effect of UVA. Photocatalytic disinfection of bacteria is mainly caused by oxidative radicals released from irradiated TiO2 (Benabbou et al. 2007). Concerns on the impact of irradiated TiO2 present in water have resulted in this research focusing on the search for the role of TiO2 in removal of bacteria. Previous study presented by Benabbou et al. (2007) indicated that E. coli was irradiated by UVA (340 nm) for 5 h without TiO2, and none of the E. coli inactivation was observed. The results of our study indicate that the removal efficiency of E. coli in the presence of TiO2 (93.5%) was better than in the absence of TiO2 (76.0%) after 30 min irradiation (Fig. 1). Also, compared with the absence of TiO2 with 78.3% bacterial removal, irradiation with TiO2 has higher bacterial removal efficiency of 100% for S. aureus. Accordingly, our findings suggest that the presence of TiO2 was critical in the process of photocatalysis in removal of bacteria, and the removal efficiency of S. aureus was more effective than that of E. coli. In terms of removal mechanisms, Rincón and Pulgarin (2003) indicated that in the presence of TiO2, the UV irradiation enhanced ·OH generation, inhibiting the self-protection mechanisms of the bacteria.
Photocatalytic Escherichia coli inactivation in the presence or absence of TiO2. The coated TiO2 concentration was 15 mg on the Petri dish. Initial concentration of E. coli: 107 CFU/mL; light intensity was 3.4 × 10−5 Einsteins s−1; 35°C (error bars represent standard error from mean for triplicates)
3.2 Interruption of Illumination
From the point of energy saving, a short time (1, 2, and 5 min) interruption of illumination during photocatalysis was conducted to evaluate the influence of intermittent irradiation to disinfect the E. coli with TiO2. Figure 2 shows the increasing effect of 1 and 2 min interruptive time of illumination on bacterial removal could be clearly observed from the removal curves with scale of 10 min intermittent irradiation. The initial decreasing accounts for the insignificant removal efficiency (<3%) on TiO2 seen in intermittent irradiation when 1 and 2 min interruptive time of illumination were used. In terms of 5-min interruptions of illumination, the beginning of the decreasing in removal efficiency was observed in the first 25 min and achieved 99.8% removal capability after 45 min treatment. This significant decrease in removal efficiency was mainly caused by the self-protection mechanisms of the bacteria. This finding corresponds with the mechanism of catalytic oxidation for bacterial removal presented by Gourmelon et al. (1994). According to this mechanism, the synthesis of the superoxide dismutase enzymes constitutes one of the major defense mechanism of cells against oxidation stress by catalyzing the disproportionation of superoxide anions O2·– (precursors of ·OH) into hydrogen peroxide and molecular oxygen. This response to oxidative stress has been well characterized for some microorganisms and especially for the facultative aerobic Gram-negative bacteria as E. coli (Gourmelon et al. 1994). Stopping irradiation at the beginning of photocatalysis favors the autorepairing mechanisms and consequently increases the required time to complete disinfection. A similar prolongation of illumination to reach the complete photocatalytic disinfection was observed by Laot et al. (1999) with the bacterium Bacteriodies fragilis, when the irradiation was interrupted during 6 min. However, Pham et al. (1995) also reported that intermittent illumination reduced viable Bacillus pumilus spores more effectively than continuous exposure to UV. Our findings suggest that the response of the microorganisms towards the intermittency of the irradiation may vary for each organism. We therefore conclude that the determination of providing the minimum illumination time (intermittent or continuous) for various microorganisms is critical for inducing an irreversible bacterial inactivation.
Effect of intermittent illumination on removal efficiency of Escherichia coli by photocatalysis. The coated TiO2 concentration was 15 mg on the Petri dish. Initial concentration of E. coli: 107 CFU/mL; light intensity was 3.4 × 10−5 Einsteins s−1; 35°C (error bars represent standard error from mean for triplicates)
3.3 Effect of Adsorption by Fixed TiO2
Figure 3 shows the removal efficiency of fixed TiO2 for both selected bacteria increased linearly at initial 30 min treatment and changed insignificantly from 30 to 90 min. In general, the fixed TiO2 demonstrated higher E. coli adsorption capability than S. aureus. In accordance with the results presented in Fig. 3, the optimal removal capability of the fixed TiO2 for E. coli and S. aureus correlates highly with the stabilized efficiency at 20.8% and 16.2%, respectively. However, in contrast to the system with UV irradiation, TiO2 alone had very little effect on adsorptive capability. Previous study conducted by Rincón et al. indicated that E. coli survived in the presence or absence of TiO2 suspended after 90 min treatment, and no adsorption of E. coli were observed. (Rincón et al. 2001; Rincón and Pulgarin 2003). We therefore conclude that the adsorption of E. coli by TiO2 coated on Petri dish is much better than TiO2 in suspension.
Effect of adsorption on removal efficiency of Staphylococcus aureus and Escherichia coli by TiO2. The coated TiO2 concentration was 15 mg on the Petri dish. Initial bacteria concentration: 107 CFU/mL; light intensity was 3.4 × 10−5 Einsteins s−1; 35°C (error bars represent standard error from mean for triplicates)
3.4 Effect of Fixed TiO2 Amount
Previous research on the photocatalytic degradation of organic compounds has suggested that the optimal amount of TiO2 is closely associated with the nature of the compounds and the reactor geometry (Gimenez et al. 1999). In terms of photocatalytic degradation of organic substances, the optimal TiO2 concentration in suspension ranged from 0.1 to 5 g/l as reported by Rincón et al. (2001). In our study, the influence of TiO2 concentration on E. coli inactivation was investigated in the range of 0.3–4.0 g/l (corresponding to 4.0–60 mg coated on the glass Petri dish). Data from Fig. 4 indicated the existence of the catalyst at 0.3 and 1.0 g/l with optimal efficiency in removing S. aureus and E. coli, respectively. This finding indicates that the antibacterial effect of the TiO2 is not necessarily related with the higher amounts/concentrations of the catalyst. Based on the experimental data, we suggest that the optimal concentration of TiO2 is 15 mg. This result corresponds with the finding of TiO2 concentration obtained in the effluent of a biological wastewater treatment plant using the same initial bacteria concentration of 107 CFU/ml (Rincón and Pulgarin 2003).
3.5 Effect of the Initial Bacteria Concentration
To further exploit and better understand the sterilization efficiency by which bacteria are removed by catalyst in photocatalytic process, we looked at the critical effect of initial bacteria concentration on bacterial removal. Figure 5 shows the influence of various initial bacteria concentration for their efficiency to remove S. aureus and E. coli. The removal efficiency of initial bacteria concentration increased from the lowest concentration of 105 CFU/mL to the highest concentration of 109 CFU/mL in both S. aureus and E. coli. This finding, which is agreement with the results reported by Rizzo (2009), who tested the different concentrations of E. coli in photocatalysis, suggests that the high concentration of 7.8 × 106 CFU/mL does not always promises a satisfactory disinfection of E. coli. Also, previous research on the photocatalysis with TiO2 has suggested that the occurrence of small ratio ·OH/bacteria in the suspension usually cause the less efficiency in the disinfection process (Bekbolet 1997). Accordingly, the disinfection system used in this study showed a promising application in low to medium bacteria polluted aqueous environment, with optimal polluted bacteria concentration below 109 CFU/mL.
Effect of initial bacteria concentration on removal efficiency of Staphylococcus aureus and Escherichia coli by photocatalysis. The coated TiO2 concentration was 15 mg on the Petri dish. Light intensity was 3.4 × 10−5 Einsteins s−1; 35°C (error bars represent standard error from mean for triplicates)
3.6 Effect of Turbidity
Turbidity in water is caused by suspended matter such as clay, silt, organic matter, plankton, and other microscopic organisms that interfere with the passage of light through the water depending on the size, number, shape, refractive index of the particles, and the wavelength of the incident light (Rincón and Pulgarin 2003). Figure 6 shows the effect of the turbidity on removal efficiency after 30 min of treatment, with a significant decreasing on bacteria removal at 100 nephelometric turbidity units (NTU). Also, results indicated that the effect of bentonite on bacterial removal was higher than that of kaolin. This finding corresponds with the fact of scattered structure of bentonite which presents more effect on bacterial removal. In terms of removal mechanism, previous research on a biological wastewater treatment plant has suggested that this decreasing removal efficiency usually occur while the turbidity increases from 27 to 84 NTU (Rincón and Pulgarin 2003). The increased turbidity of water reducing the photocatalytic inactivation rate of bacteria suggested two possible reasons: first, organic particles compete with bacteria for both oxidative ·OH radicals and the photoactive sites of TiO2; second, particles induce a reduction of solar penetration through turbid water by the scatter effect. Accordingly, it is suggested that higher concentration of bentonite and kaolin particles might reduce the solar penetration effect. For the first time, this research demonstrates the evaluation on the bacteria removal efficiency with various sources of turbidity agents in photocatalysis.
Effect of turbidity on the photocatalysis of Staphylococcus aureus and Escherichia coli inactivation after 30 min of irradiation. The coated TiO2 concentration was 15 mg on the Petri dish. Initial bacteria concentration: 107 CFU/mL; light intensity was 3.4 × 10−5 Einsteins s−1; 35°C (error bars represent standard error from mean for triplicates)
3.7 Effect of Coexisting Organic Matters
The polymer or copolymer is one which can be degraded by accelerated photodegradation. Most organic polymers display some sensitivity to photo-oxidative degradation. Also, organic compounds existing in the solution might interfere in the disinfection reaction of the photodegradation. Thus, the photoformation of ·OH free radicals or chemical bonds in organic compounds is the major cause of the difference in photodegradation rates (Rincón et al. 2001). A simple organic compound, glucose, has been selected as the sample. Figure 7 shows that the removal efficiency decreased with the increase of glucose concentration from 0.001% to 0.1%. A similar trend was found for both S. aureus with 99.2% to 90.3% and E. coli with 89.3% to 80.3%. Our findings clearly reveal that glucose with high concentration decrease the generation of free radicals in the solution, and the removal efficiency decreased with increasing concentration of glucose.
Effect of coexist organic matter on the photocatalysis of Staphylococcus aureus and Escherichia coli inactivation after 30 min of irradiation. The coated TiO2 concentration was 15 mg on the Petri dish. Initial bacteria concentration: 107 CFU/mL; light intensity was 3.4 × 10−5 Einsteins s−1; 35°C (error bars represent standard error from mean for triplicates)
3.8 Comparison of Synthetic Water and Crude Water
To further validate the application of the designed disinfection method, bacteria removal efficiency between synthetic water and crude water was investigated and compared. Table 1 shows the concentrations of S. aureus and E. coli with 30 min photocatalysis in synthetic/crude water remained unchanged after 12 and 24 h analysis by cell counting. This finding indicates that the bacteria have been disinfected during the photocatalysis process, and UV irradiation function alone has less influence in removal of microorganisms in the water. Accordingly, disinfection of bacteria in synthetic or crude water with UV irradiation alone provides more chance for bacteria to exist and re-grow in the medium. In terms of bacteria removal in photocatalysis, system in synthetic water performs much better than that of crude water. The possible high removal capability for system in synthetic water is caused by the unknown surrounding environmental factors existing in the crude water. Previous studies presented by Wist et al. (2002) and Svensäter et al. (2000) showed possible removal mechanism, in which the UV irradiation might provide new sources of nutrients released from dead cells or by action on the present biomass and organic compounds. Thus, some bacteria show more resistance than others that lost their ability to reproduce during the UV irradiation without TiO2. Accordingly, further exploration of the influence from surrounding environmental factors for photocatalysis in crude water will be carried out in future studies.
4 Conclusions
The results of this study demonstrate that photocatalysis with fixed TiO2 method was effective and promising in bacteria inactivation. However, in terms of removal efficiency, the adsorption with fixed TiO2 has performed much worse than the process with direct UV irradiation and photocatalysis. The interruption of illumination test further confirmed that no bacterial cultivability has been recovered after 24 h observation, indicating the durability of the sterilization by photocatalysis. Our findings also indicate that the 5-min interruption of illumination would significantly decrease the bacteria removal efficiency.
References
Ao, C. H., Leung, M. K. H., Lam, R. C. W., Leung, D. Y. C., Vrijmoed, L. L. P., Yam, W. C., et al. (2007). Photocatalytic decolorization of anthraquinonic dye by TiO2 thin film under UVA and visible-light irradiation. Chemical Engineering Journal, 129, 153–159.
Bekbolet, M. (1997). Photocatalytic bactericidal activity of TiO2 in aqueous suspensions of E. coli. Water Science and Technology, 35, 95–100.
Benabbou, A. K., Derriche, Z., Felix, C., Lejeune, P., & Guillard, C. (2007). Photocatalytic inactivation of Escherischia coli effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Applied Catalysis. B, Environmental, 76, 257–263.
Cushnie, T. P. T., Robertson, P. K. J., Officer, S., Pollard, P. M., McCullagh, C., & Robertson, J. M. C. (2009). Variables to be considered when assessing the photocatalytic destruction of bacterial pathogens. Chemosphere, 74, 1374–1378.
Gelover, S., Gomez, L. A., Reyes, K., & Leal, M. T. (2006). A practical demonstration of water disinfection using TiO2 films and sunlight. Water Research, 40, 3274–3280.
Gimenez, J., Curco, D., & Queral, M. A. (1999). Photocatalytic treatment of phenol and 2, 4-dichlorophenol in a solar plant in the way to scaling-up. Catalysis Today, 54, 229–243.
Gourmelon, M., Cillard, J., & Pommepuy, M. (1994). Visible light damage to Escherichia coli in seawater: Oxidative stress hypothesis. Journal of Applied Bacteriology, 77, 105–112.
Hijnen, W. A. M., Beerendonk, E. F., & Medema, G. J. (2006). Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Research, 40, 3–22.
Kim, B., Kim, D., Cho, D., & Cho, S. (2003). Bactericidal effect of TiO2 photocatalyst on selectedfood-borne pathogenic bacteria. Chemosphere, 52, 277–281.
Kuhn, K. P., Chaberny, I. F., Massholder, K., Stickler, M., Benz, V. W., Sonntag, H. G., et al. (2003). Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere, 53, 71–77.
Laot, N., Narkis, N., Neeman, I., Bilanovic, D., & Armon, R. (1999). TiO2 photocatalytic inactivation of selected microorganisms under various conditions: Sunlight, intermittent and variable irradiation intensity, CdS augmentation and entrapment of TiO2 into sol-gel. Journal of Advanced Oxidation Technologies, 4, 97–102.
McCullagh, C., Robertson, J. M. C., Bahnemann, D. W., & Robertson, P. K. J. (2007). The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: A review. Research on Chemical Intermediates, 33, 359–375.
Pham, H. N., Mcdowell, T., & Wilkins, E. (1995). Photocatalytically-mediated disinfection of water using tio2 as a catalyst and spore-forming Bacillus pumilus as a model. Journal of Environmental Science and Health, Part A, 30, 627–636.
Rincón, A. G., & Pulgarin, C. (2003). Photocatalytical inactivation of E. coli: Effect of (continuous–intermittent) light intensity and of (suspended–fixed) TiO2 concentration. Applied Catalysis. B, Environmental, 44, 263–284.
Rincón, A. G., Adler, N., Pulgarin, C., & Peringer, P. (2001). Interaction between E. coli inactivation and DBP-precursors—dihydroxybenzene isomers—in the photocatalytic process of drinking-water disinfection with TiO2. Journal of Photochemistry and Photobiology. A, Chemistry, 139, 233–241.
Rizzo, L. (2009). Inactivation and injury of total coliform bacteria after primary disinfection of drinking water by TiO2 photocatalysis. Journal of Hazardous Materials, B165, 48–51.
Svensäter, G., Sjögreen, B., & Hamilton, I. R. (2000). Multiple stress responses in Streptococcus mutans and the induction of general and stress-specific proteins. Microbiology, 146, 107–117.
Wist, J., Sanabria, J., Dierolf, C., Torres, W., & Pulgarin, C. (2002). Evaluation of photocatalytic disinfection of crude water for drinking-water production. Journal of Photochemistry and Photobiology. A, Chemistry, 147, 241–246.
Acknowledgments
The work was supported by Grant from the National Science Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, CY., Wu, LC., Chen, HY. et al. Inactivation of Staphylococcus aureus and Escherichia coli in Water Using Photocatalysis with Fixed TiO2 . Water Air Soil Pollut 212, 231–238 (2010). https://doi.org/10.1007/s11270-010-0335-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0335-y