Abstract
Mercury pollution in the Second Songhua River (SSR) was serious in the last century due to effluent from a chemical corporation. Effects of riverine self-purification on mercury removal were studied by comparing monitoring data of mercury concentrations varieties in water, sediment, and fish in the past, about 30 years. The present work suggested that a river of such a size like the SSR possessed the potential ability to recover from mercury pollution under the condition that mercury sources were cut off, though it needs a very long time, which might be several decades or even a century of years. During the 30 years with no effluent containing mercury input, total mercury (T-Hg) of water and sediment in some typical segments, mostly near the past effluent outlet, had decreased radically but still higher than the background values, though the decrease amplitudes were over 90% compared with that in 1975. T-Hg had decreased by more than 90% in most fishes, but some were still not suitable for consumption. Methylmercury concentrations (MeHg) of water, sediment, and fish were higher or close to the background levels in 2004. In the coming decades, the purification processes in the SSR would be steady and slow for a long period.
Similar content being viewed by others
1 Introduction
Mercury is toxic and persistent in the environment. Mercury, especially methylmercury, is a neurotoxin that affects human health via bioaccumulation through the food chain in aquatic systems (Hylander and Goodsite 2006). Methylmercury is biomagnified in aquatic food webs, and top predatory fish generally are the most highly contaminated (Kehrig et al. 2008). There are potential adverse health implications for both these fish and animals that consume them. High mercury levels in the body would lead to Minamata disease, which has been reported frequently since industrial revolution in the last middle century.
It is a world concern and an important subject whether water would be purified and recover from mercury contamination just depending on natural processes in rivers; it is especially meaningful for large rivers. Though a number of papers have published in the general areas of mercury fate in rivers, lakes, and reservoirs (Hope and Rubin 2005; Gosar et al. 1997; Kannan et al. 1998), little work has been carried out on efficiencies of mercury removal just depending on the natural processes during a long period. These natural processes were defined as the river self-purification including mercury migration and transport in sediments; volatilization to the atmosphere from water surface; transport by water mass; fixation by some groups and ions like the S2−; and absorption by phytoplankton, zooplankton, and fish. In lake Vänern, Sweden, the previous work on mercury recovery from contamination indicated that mercury content in lake sediments in 2001 had only decreased by slightly more than a half since the mid-1970s, despite a radical decrease in effluents from the source during the 1960s (Lindeström 2001; Danielsson et al. 2002). Mercury pollution in water bodies could last for many years, for example, mercury in Soca/Isonozo was not as low as expected even 10 years later after the Idrija mine, the mercury source, was closed (Horvat et al. 1999). In Yatushiro Sea, mercury was released into the cover water instead of being fixated by sediment even more than 30 years later since the mercury sources were cut off (Tomiyasu et al. 2000). It indicated that natural processes might not be as effective as expected on mercury removal. Characteristics of mercury remediation in water by river self-purification are yet unknown, and there is a lack of study.
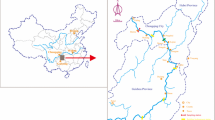
The Second Songhua River (SSR) is the largest tributary of the Songhua River, northeast China. In the last century, the SSR was seriously polluted by mercury (Wang 1977). There were several investigations on mercury levels in the SSR in 1970s, 1980s, 1990s, 2003, and 2004; and one of the authors had participated all sampling and analyzing working. During 2003–2004 and 2006–2007, we investigated the present situations of mercury pollution twice in the SSR and studied the characteristics of mercury removal by river self-purification since 1982 when the mercury sources were cut off.
2 Materials and Method
2.1 Site Description
The SSR was seriously polluted by mercury mostly because of effluents discharged from the Jilin Chemical Industry Corporation (JCIC) in the last century (Wang 1977). From 1958 to 1982, the JCIC produced acetaldehyde using HgSO4 as the activator and generated large amounts of effluent, which were discharged into the SSR without any treatments to remove mercury and methylmercury (MeHg). At the peak periods of mercury input, T-Hg of water was about 400 times the background value, and the highest T-Hg of sediment was more than 1,000 mg/kg (Wang et al. 1982). Channels with T-Hg over than 1.0 mg/kg in surface sediment extended to hundreds of miles (Wang et al. 1990; NEIGAE 1994). Symptoms of Minamata disease, which just had been reported in Japan before, had appeared in fishermen who consumed much fish (Bao et al. 1982). In 1982, the JCIC reformed its technology for acetaldehyde production, and HgSO4 was not used anymore. Since then, no more mercury was discharged into the SSR from the JCIC. Mercury release from sediment has become the main mercury source in the channels from Shihaoxian to Ganshuigang (Wang et al. 1985, 2007).
2.2 Segments and Sampling
Segments were set as the same as these investigated in 1970s and1980s (Fig. 1). Locations of segments were signed and affirmed by bridges, ferries, landforms, and other markers because the global positioning system technology was not used in the previous investigations. During 2003–2004 and 2006–2007, water and sediment samples were collected simultaneously. pH of water samples was adjusted to 1.0 with HNO3 immediately and preserved in polyethylene bottles. Sediment was sealed in polyethylene bags and preserved at 4°C.
In 2003–2004, major fish species were collected in the SSR, and fish body weight and length were measured in situ. Fish and mussels were brought back with polyethylene bags containing ice in and preserved at −20°C in laboratory. For mercury analysis, fish was washed with deionized water, and muscle tissue in fish back was used for mercury analysis.
2.3 Chemical Analysis
2.3.1 T-Hg in Water, Sediment, and Fish
For T-Hg determination in water, KMnO4 and K2S2O4 were used for digestion (Wang et al. 1990). Sediment was dried at room temperature, ground to pass an 80-mesh nylon sieve. Sediment and fish muscles were digested using the HNO3-H2SO4-V2O5 method (Liu et al. 2003). The cold atomic absorption technology was used for T-Hg determination by an F732-V mercury detector. The analysis methods were the same as that used in 1970s and 1980s.
Precision and accuracy of the analytical method were evaluated by comparing the expected T-Hg in certified reference materials to the measured values. The expected and measured concentrations in reference material (GBW-07401) were 0.032 ± 0.006 and 0.031 ± 0.004 mg/kg, respectively.
2.3.2 MeHg Analysis in Water, Sediment, and Fish Muscles
MeHg in sediment was extracted and separated using the method described by Zhang et al. (2009). Sediment (0.5 g) was weighed into a 50-mL polyethylene centrifuge tube. About 5 mL 6 mol/L HCl was added to extract all forms of mercury. The tube was placed overnight and then was ultrasonically cleaned for 2 h by the Ultrasonic Cleaner Instrument. After that, the tube was centrifuged at 3,000 rpm for 15 min. The supernatant fluid was transferred into a 10-mL polyethylene centrifuge tube; 2 mL of CH2Cl2 was added and shaken for 1 h to extract organomercury compounds into the CH2Cl2 phase. After centrifuging at 3,000 rpm for 15 min, the CH2Cl2 phase was transferred into a 50-mL glass tube; an additional 2 mL of CH2Cl2 was added into the 10-mL polyethylene centrifuge tube again to remove any remaining MeHg. The same steps were repeated on the same sediment sample. Finally, 5 mL deionized water was added into the 50 mL glass tube, which had contained the 4-mL CH2Cl2 phase. The 50-mL glass tube was place in a 60°C water bath and was aerated by N2. The CH2Cl2 was blown off, and the organomercury compounds were left in the water phase. Finally, 1 mL 1:1 18 mol/L H2SO4 and 1 mL bromide agent (KBrO3 + KBr) were added into the 50-mL glass tube, which played the role of oxidation and indicator, respectively. After 1 h, a drop of hydroxylamine hydrochloride was added into the tube to deoxidize the residual bromide agent. The MeHg was determined by the Tekran Model 2600, which was stable and the recoveries of MeHg were between 94.1% and 102.3%.
MeHg in water was extracted directly using the CH2Cl2, and the following procedures were the same as mentioned above.
MeHg in fish was extracted and determined according to the national standard method of China (GB/T 17132-1997): 1.0–2.0 g fish muscle was weighed and ground in a bowl with NaCl added. MeHg was thoroughly extracted out using 2.0 mol/L and then enriched on the sulfhydryl cotton, washed off using 2.0 mol/L HCl, and extracted using benzene at last. MeHg in the benzene was analyzed with the Gas chromatography 2010 (Shimadzu, Japan).
All of the glass vessels was soaked overnight in 30% HNO3, rinsed with copious amounts of distilled deionized water, stored, capped, and filled with deionized water, prior to use. All solutions were prepared with distilled deionized water in glass bottles and handled with analytical micropipettes. SPSS 10.0 for windows and Excel 2003 were sued for data analysis; Arcgis 9.0 and Origin 7.5 were used for mapping.
3 Results
3.1 Mercury Input into the SSR during 1958–1982
Mercury pollution in the SSR could be traced back to the 1950s when chemical industries developed quickly in China. Mercury input into the SSR was composed of four parts: (1) mercury from the soil and rock by dissolution, which contributed to the mercury background levels in the SSR sediment and water; (2) mercury brought into the SSR by surface runoff, and this part was estimated to about 1.7 t/a, of which about 1.05t was precipitated in the channels; (3) mercury input via atmospheric wet and dry processes, and this part was about 0.1 t/a on the entire SSR catchment and about 40% was precipitated in the SSR; and (4) mercury from anthropogenic sources, mostly input by effluent discharged from plants producing acetaldehyde, polyethylene, and calcium carbide of the JCIC. About 149.8 t mercury and 5.4 t methylmercury were estimated to discharge into the SSR from anthropogenic sources, and the JCIC accounted for about 94% of T-Hg and 100% of MeHg (NEIGAE 1994).
From 1958 to 1982, rate of MeHg input into the SSR was 665 g/day. The period when rate of T-Hg input was over 1,000 g/day was about 7 years, and the rate was over 1,500 g/day from 1973 to 1975. In the peak periods, about 30.3 kg mercury was discharged into the SSR everyday (NEIGAE 1994). In 1982, HgSO4 was not used anymore. Since then, mercury was purified mostly by natural processes in the SSR.
3.2 Mercury in Sediment
In the past 30 years, total organic carbon (TOC) contents of sediments varied slightly in Shihaoxian, Hadawan, and Songhuajing village (Table 1). It suggested that the sediments were comparable over time.
In the past, the highest T-Hg in surface sediment was found in Shihaoxian. T-Hg in surface sediment was very high before 1983 but decreased radically since mercury sources were closed (Fig. 2). T-Hg in the surface sediment (<10 cm) of typical sediments, Shihaoxian, Hadawan, and Baiqi, were 1.12, 0.219, and 0.475 mg/kg in 2006, which had decreased by 93.4%, 97.2%, and 69.2% compared to T-Hg in 1983, respectively, but T-Hg was still much higher than the background level, which was just 0.036 mg/kg.
Mercury in the surface sediment was mainly removed by two potential routes. One was that surface sediment with extreme high mercury concentrations were transported downstream by the water flow, which led to the mercury levels rising in downstream; another was that the new precipitation with low mercury concentrations from upstream had diluted the mercury concentrations in some segments heavily polluted like Shihaoxian. Mercury distribution characteristics in vertical direction indicated that T-Hg in surface sediment was higher than the deeper layers in 1970s, but it was lower than the deeper layers in 2004 (Fig. 3). It suggested that the relatively higher T-Hg in deeper layers might be the secondary mercury source.
MeHg background level in sediment of the SSR was 0.25 μg/kg. In the period of 1976–1978, MeHg concentrations were extremely high, which mostly came from effluent discharged directly from the JCIC. MeHg in the Shihaoxian segment was more than 100 μg/kg, which was about 400 times the background level. In 1982, mercury sources were cut off, and no more MeHg was discharged into the SSR via effluent; since then, MeHg in the sediment was formed mostly by methylation processes stimulated by microorganisms. From 1983 to 2004, MeHg of sediment was decreasing in segments near the Shihaoxian, but increasing in some segments downstream. In 2004, MeHg in Shihaoxian was 3.84 μg/kg, which had decreased by about 96% in the past 30 years.
3.3 Mercury in Water
T-Hg background level in water of the SSR is 0.03 μg/L (Shao 2004). In 2006, T-Hg in water ranged from 0.009 to 0.069 μg/L, and the average was 0.033 μg/L, which was close to the background level. From 1975 to 2006, though T-Hg in water had decreased by 99.8% in Shihaoxian, 98.9% in Baiqi, and 97.0% in Shaokou, respectively (Fig. 4), it was still higher than the background levels in segments near Shihaoxian, which suggested the historical influences of the JCIC.
In 1983, MeHg in water decreased radically than that in 1981, but MeHg in water was higher in 2007 than that in 1983 (Fig. 4). MeHg in the SSR mostly came directly from effluent from the JCIC in 1981, when mercury sources were cut off, with no exogenous MeHg input directly; MeHg in water was very low in 1983. MeHg rising in water in 2007 might be due to MeHg dissolving in cover water from mercury methylation stimulated by microorganisms in sediment.
3.4 Mercury in Fish and Mussels
Since most studies have identified a clear association between quantity and the frequency of fish consumption and Hg poison, mercury in fish has been paid extraordinary attention. We had tested the correlationship between T-Hg and fish body length and weight of the Parasiburus asvtus, Hemiculter leucisculus, Carassius auratus gibeliv, Cyprinus carpio Linnaeus, and Saurogobio dabryi. Results suggested that significantly positive correlationships between T-Hg and fish body length and weight were only found in the C. auratus gibeliv (Fig. 5). Some researches indicated that T-Hg was related to fish body length and weight because that reflected the age of fish indirectly, but our results suggested the positive correlationships were not found in most kinds of fish, which might be due to results from the joint effects of mercury half-time and growth rate in different fish species. Another potential reason was that fish in river did not stay in the stationary channels, and they had larger range of motion than that in lakes and reservoirs. In 1976, the investigation also suggested no significantly relationship between T-Hg and fish body weight, length, and age.
In 2004, fish body lengths investigated were in the range of 6.6–24.3 cm, and the average was 13.54 cm, which did not vary too much with that measured in the past. It suggested that T-Hg in fish of different sizes were comparable.
From 1975 to 1978, T-Hg in 26 fish species of the SSR was thoroughly investigated and ranged from 0.03 to 3.240 mg/kg (wet weight) with the average 0.744 mg/kg. Of the 574 fish samples determined, about 63.4% was not suitable for consumption. The highest T-Hg was observed in Lampetra reissneri, which was the endangered species in the SSR, and was in the range of 1.480–3.240 mg/kg with the average 2.071 mg/kg. MeHg of fish ranged from 0.22 to 1.620 mg/kg and accounted for about 59% of the T-Hg (NEIGAE 1994).
The predominant fish species in the SSR were Carassiu auratus, C. carpio, Xenocypris macrolepis, Hypophthalmichthys molitrix, H. leucisculus, and P. asvtus. Of the five fish species, T-Hg ranged from 0.032 to 2.375 mg/kg in 1975, which was much higher than the guide mercury limit of China (Table 2).
Sixty-four fish samples were chose to analyze the MeHg in 1977. Results indicated that MeHg was significantly related with T-Hg (r = 0.976, p < 0.001). Of the 12 fish species investigated, MeHg in Lota lota accounted for 77% of T-Hg, and the proportion was higher than the other species (Table 3).
From 2003 to 2004, another investigation on the mercury concentrations in fish (ten species, 158 fish samples) and mussels (28 samples) were performed, and results suggested that T-Hg ranged from 0.001 to 0.66 mg/kg with the average 0.139 mg/kg (Fig. 6), which was lower than the guide mercury limit of China (0.3 mg/kg). The highest T-Hg was observed in Carassius auratus with the average 0.45 mg/kg (Shao 2004).
T-Hg of fish in 2004 had decreased radically by 91.4% compared with those in 1975. Of the representative fish species, the amplitude of decreasing from 1975 to 2004 ranged from 78.9% to 98.0%, which suggested that mercury had been removed from fish effectively (Fig. 6). T-Hg in four mussel species, Anodonta woodiana woodiana, Cristaria plicata, Lanceolaria grayana, and Unio douglasiae, were investigated, and it was also much lower than that in 1975 (Table 4).
4 Discussion
4.1 Mercury Recovery Model from Contamination in Sediment
Four segments, Shaokou, Baqi, Fuyu, and Ganshuigang, were chosen to study the mercury recovery model in the surface sediment just depending on river self-purification processes since 1983. Regression analysis indicated that T-Hg of all sections were negative exponential with purification time (Table 4). It suggested that T-Hg in the surface sediment decreased significantly with large gradient in the first several years after the pollution sources were cut off (Fig. 7 and Table 5); and 8–10 years later, T-Hg decreased slowly, and the model curve became smooth and steady. Mercury removal efficiency was depending on the initial T-Hg in sediment in 1983. On the whole, the higher the initial T-Hg was, the faster the purification rate was. The model indicated that mercury purification rate was slow in the future and would continue for a long period.
4.2 Mercury Recovery from Contamination in the Water
All water samples were collected in the normal river flow period. It suggested that T-Hg of water in Shaokou, Baiqi, and Chaoyang were all exponential negative with time (Fig. 8), but Fuyu was obviously different with the other three segments. In 1990s, T-Hg of water in Fuyu was increasing in the first several years, and the reasons were unclear.
4.3 Mercury Recovery from Contamination in Fish
In general, mercury was persistent in fish and mostly in methylmercury forms. Due to the biomagnification of mercury along the food chain, the highest mercury concentrations were usually found in carnivores. From 1973 to 2003, T-Hg in fish collected from most segments, though were decreasing and had decreased by 91.4% on the whole compared with those in 1975 (Fig. 9), were still very high and were about 1.6–6.9 times of the background value. In 1976, T-Hg in fish of the SSR was higher than those had reported in the Minamata bay in 1972, which was 0.73 mg/kg on average. Of all fish species investigated, T-Hg was in the order of the carnivorous > omnivorous > herbivorous, the demersal > the pelagic and the alepidote > the lepidote. Up to the present, some fish species were still not suitable for consumption.
Some models proposed in the 1990s had predicted that mercury pollution in water, sediment, and fish of the SSR would reduce to the background values after 10–20 years later since mercury sources were closed. Results of the present work showed that the model was over-optimistic (Wang 1992; Yu et al. 1994). Effects of mercury purification in the SSR just depending on the river natural processes were not as good as results expected. It suggested that the steady and low mercury-removing rate will last several decades or even more than hundreds of years before recovery from contamination.
5 Conclusions
The present work suggested that mercury concentrations in the SSR was dominated by mercury input from effluent before mercury sources were closed in 1982, and the river purification was predominant since 1983 and resulted in mercury concentration decreasing in the past 30 years. It was estimated that about 149.8 t mercury and 5.4 t MeHg were discharged into the SSR from 1958 to 1982 in all. In 2004, T-Hg in water and sediment near the past effluent outlet, the Shihaoxian, had decreased by 90%. The mercury-removing rates in surface sediment depended on and increased with initial T-Hg in 1983. It can be concluded that from the vertical T-Hg distribution characteristics in 1976 and 2004, purification processes mostly occurred in surface sediment with no more than 40 cm depth. T-Hg in most fishes investigated had decreased by over 90% in 2004 compared with 1975, but still were high and about 1.6–6.9 times of the background values. Some representative fishes were not suitable for consumption yet. From 1983 to 2007, T-Hg in water, sediment, and fish were decreasing with times in a potential negative exponent model. Since mercury sources were cut off, T-Hg in water, sediment, and fish decreased firstly radically in the initial 10 years and then slow in the following time. Regression analysis suggested that it would take about several decades or more than a hundred of years in the future to recover from mercury concentrations to the background values in the SSR.
References
Bao, L. P., Lin, X. W., & Hou, Z. T. (1982). The potential effects of methyl mercury exoposed by intermittently eating a great lot of fishes polluted by mercury in the Songhua River. Environmental Science, 3, 42–44. (in Chinese).
Danielsson, Å., Wihlborg, P., & Rahm, L. (2002). Large-scale mercury variations in Lake Vänern (Sweden) 1974–98. Environmental Monitoring and Assessment, 75, 223–234.
Gosar, M., Pirc, S., & Bidovec, M. (1997). Mercury in the Idrijca River sediments as a reflection of mining and smelting activities of the Idrija mercury mine. Journal of Geochemical Exploration, 58, 125–131.
Hope, B. K., & Rubin, J. R. (2005). Mercury levels and relationships in water, sediment, and fish tissues in the Willamette Basin, Oregon. Archives of Environmental Contamination and Toxicology, 48, 367–380.
Horvat, M., Covelli, S., Faganeli, J., et al. (1999). Mercury in contaminated coastal environments: a case study: the Gulf of Trieste. Science of the Total Environment, 237–238, 43–56.
Hylander, L. D., & Goodsite, M. E. (2006). Environmental costs of mercury pollution. Science of the Total Environment, 368, 352–370.
Kannan, K., Smith, R. G., Lee, R. F., Windom, H. L., Heitmuller, P. T., Macauley, J. M., et al. (1998). Distribution of total mercury and methyl mercury in water, sediment, and fish from South Florida Estuaries. Archives of Environmental Contamination and Toxicology, 34, 109–118.
Kehrig, H. A., Howard, B. M., & Malm, O. (2008). Methylmercury in a predatory fish (Cichla spp.) inhabiting the Brazilian Amazon. Environmental Pollution, 154, 68–76.
Lindeström, L. (2001). Mercury in sediment and fish communities of Lake Vänern, Sweden: Recovery from contamination. AMBIO, 30, 538–544.
Liu, R. H., Wang, Q. C., Lv, X. G., et al. (2003). Distribution and speciation of mercury in the peat bog of Xiaoxing'an Mountain, northeastern China. Environmental Pollution, 124, 39–46.
Northeast Institute of Geography and Agricultural Ecology, Chinese Academy of Sciences (NEIGAE) (1994). A comprehensive reports on mercury pollution and remediation in the Second Songhua River (pp. 15–18). Changchun: Northeast institute of Geography and Agricultural Ecology. (in Chinese).
Shao, Z. G. (2004). Mercury purification and predict in the Second Songhua River. Beijing: Graduate University of Chinese Academy of Sciences. (in Chinese).
Tomiyasu, T., Nagano, A., Yonehara, N., et al. (2000). Mercury contamination in the Yatsushiro Sea, South-western Japan: spatial variations of mercury in sediment. Science of the Total Environment, 257, 121–132.
Wang, R. H. (1977). The status quo and evolving tendency of mercury pollution in the Second Songhua River. Environmental Science Information, 5, 6–12. (in Chinese).
Wang, R. L. (1992). Study on the predictable model of mercury concentration in sediments in the Second Songhua River. Beijing: Graduate University of Chinese Academy of Sciences.
Wang, R. H., Wangs, S. H., & Hao, T. C. (1982). Discussion on a comprehensive control plan of mercury pollution sediments of the Second Songhua River. The Editorial Committee of the Proceedings of the Second Songhua River Symposium on Environmental Science. Proceedings of the Second Songhua River Symposium on Envionmental Science (pp. 53–62). Changchun: Jilin People Press. (in Chinese).
Wang, S. H., Wang, Q. C., & Wang, R. H. (1985). Study on the law of methyl mercury emitted in sediments in fresh water. Acta Scientiae Circumstantiae, 5, 290–295. (in Chinese).
Wang, Q. C., Wang, S. H., & Wang, R. H. (1990). Stimulating study on the rate of mercury emitted into the Jilin-Fuyu segments in the Songhua River. Geological Science, 10, 365–371. (in Chinese).
Wang, Q. C., Shao, Z. G., & Zhang, L. (2007). Study on self-purification of the Second Songhua River polluted by mercury in past 20 years. Acta Scientiae Circumstantiae, 27, 474–479. (in Chinese).
Yu, C. R., Yu, H. B., & Liang, D. M. (1994). Prediction about the trend of methyl mercury pollution in fishes in the Songhua River. Ocean and Lake AVISO, 1, 68–73. (in Chinese).
Zhang, S. Q., Wang, Q. C., Cui, J. T., Sun, X. J., & Zheng, D. M. (2009). Rapid analysis of methylmercury in sediment and soil samples by ultrasonic-assisted solvent extraction coupled with inductively coupled CVAFS. Physical Testing and Chemical Analysis, (in press). (in Chinese).
Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (No. 40830535, 40803021, and 40901036), Major State Basic Research Projects of China (No. 2009CB42110303), and the Innovation Foundation of Chinese Academy of Sciences (No. KZCX3-SW-437). The authors appreciated the past work by researchers of the Northeast Institute of Geography and Agricultural Ecology, Chinese Academy of Sciences, People's Republic of China. Thanks to Dr. Song X. L., Yin X. M., and Huo L. L. for their suggestion on English grammar and syntax.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Zhang, Z.S., Sun, X.J., Wang, Q.C. et al. Recovery from Mercury Contamination in the Second Songhua River, China. Water Air Soil Pollut 211, 219–229 (2010). https://doi.org/10.1007/s11270-009-0294-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0294-3