Abstract
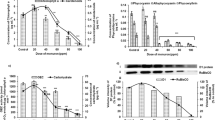
The effects of bentazon and molinate, two selective herbicides recommended for integrated weed management in rice, were studied in Anabaena cylindrica, an abundant cyanobacterium isolated from a Portuguese rice field agro-ecosystem. Comparative effects of both herbicides on A. cylindrica were estimated under laboratory conditions by measuring its dry weight yield, photopigments, and carbohydrate and protein contents in a time- and dose-dependent exposure throughout 72 h. Photosynthesis and respiration were also monitored. The results revealed that both herbicides exerted a pleiotropic effect on the cyanobacterium at the range of concentrations tested (0.75–2 mM). Growth, chlorophyll a, carotenoids and phycobiliproteins were more adversely affected by molinate than by bentazon. Cyanobacterial growth inhibitions of over 50% were observed after 48 h when 1.5–2 mM of molinate were applied. Bentazon concentrations ranging from 0.75 to 2 mM did not significantly modified chlorophyll a content with time, however, considerable reductions in chlorophyll a, carotenoids and specially phycobiliproteins were observed with molinate. Protein content increased with both herbicides although the effect was particularly noticeable with the highest concentration of molinate. Herbicide effects on carbohydrate content were contrasting: molinate increased this organic fraction whereas bentazon decrease it. Photosynthesis and respiration were inhibited by both herbicides and higher molinate concentrations (1.5–2 mM) completely ceased O2 evolution after 48 h. Since A. cylindrica is abundant in Portuguese rice fields and could be used as an inoculum source in rice biofertilization programs, their protection from potential residual effects of herbicides is fundamental for a correct management of local soil fertility.

Similar content being viewed by others
References
Ashton, F. M., & Crafts, A. S. (1981). Mode of action of herbicides (2nd ed.). New York: Wiley-Interscience.
Bagchi, S. N., Pistorius, E. K., & Michel, K.-P. (2003). A Synechococcus sp. PCC 7942 mutant with higher tolerance towards bentazone. Photosynthesis Research, 75, 171–182. doi:10.1023/A:1022836218176.
Battah, M. G., Shabana, E. F., Kobbia, I. A., & Eladel, H. M. (2001). Differential effects of thiobencarb on growth and photosynthesis of Anabaena variabilis with changes in phosphate level. Ecotoxicology and Environmental Safety, 49, 235–239. doi:10.1006/eesa.2001.2056.
Bennett, A., & Bogorad, L. (1973). Complementary chromatic adaptation in a filamentous blue-green algae. The Journal of Cell Biology, 58, 419–435. doi:10.1083/jcb.58.2.419.
Bethlenfalvay, G. J., Norris, R. F., & Phillips, D. A. (1979). Effect of bentazon, a Hill reaction inhibitor, on symbiotic nitrogen-fixing capability and apparent photosynthesis. Plant Physiology, 63, 213–215.
Böger, P., & Sandmann, G. (1998). Action of modern herbicides. In A. S. Raghavendra (Ed.), Photosynthesis: A comprehensive treatise (pp. 337–351). Cambridge: Cambridge University Press.
Buschmann, C., Grumbach, K. H., & Bach, T. J. (1980). Herbicides which inhibit photosystem II or produce chlorosis and their effect on production and transformation of pigments in etiolated radish seedlings (Raphanus sativus). Physiologia Plantarum, 49, 455–458. doi:10.1111/j.1399-3054.1980.tb03334.x.
Calha, I. M., Machado, C., & Rocha, F. (1999). Resistance of Alisma plantago aquatica to sulfonylurea herbicides in Portuguese rice fields. Hydrobiologia, 415, 289–293. doi:10.1023/A:1003880527091.
Campbell, D., Hurry, V., Clarke, A. K., Gustafsson, P., & Öquist, G. (1998). Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiology and Molecular Biology Reviews, 62, 667–683.
Carr, N. G., & Whitton, B. A. (1982). The biology of cyanobacteria. Botanical monographs (vol. 19). Oxford: Blackwell Scientific.
Castro, M., Silva-Ferreira, A. C., Manaia, C. M., & Nunes, O. C. (2005). A case study of molinate application in a Portuguese rice field: herbicide dissipation and proposal of a clean-up methodology. Chemosphere, 59, 1059–1065. doi:10.1016/j.chemosphere.2004.11.041.
Datta, S. K. (2004). Rice biotechnology: a need for developing countries. AgBioForum, 7, 31–35.
Davies, D. H. (1976). Carotenoids. In T. W. Goodwin (Ed.), Chemistry and Biochemistry of Algal Pigments. (pp. 38–165). New York: Academic.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356. doi:10.1021/ac60111a017.
Ehling-Schulz, M., & Scherer, S. (1999). UV protection in cyanobacteria. European Journal of Phycology, 34, 329–338. doi:10.1080/09670269910001736392.
Eladel, H. M., Henley, W. J., & Kobbia, I. A. (1999). Effect of thiobencarb on growth and photosynthesis of the soil alga Protosiphon botryoides (Chlorophyta). Journal of Applied Phycology, 10, 547–554. doi:10.1023/A:1008092826338.
Fernández-Valiente, E., Ucha, A., Quesada, A., Leganés, F., & Carreres, R. (2000). Contribution of N2 fixing cyanobacteria to rice production: availability of nitrogen from 15N-labelled cyanobacteria and ammonium sulphate to rice. Plant and Soil, 221, 107–112. doi:10.1023/A:1004737422842.
González Tomé, R. (1996). Estudio de diversas técnicas agronómicas en relación con las cianobacterias fijadoras de N2 en el cultivo del arroz. Ph.D Dissertation. Facultad de Ciencias Biológicas, Universidad de Valencia, Valencia (in Spanish).
Hammouda, O. (1999). Response of the paddy field cyanobacterium Anabaena doliolum to carbofuran. Ecotoxicology and Environmental Safety, 44, 215–219. doi:10.1006/eesa.1999.1823.
Hirschberg, J., & Chamovitz, D. (1994). Carotenoids in Cyanobacteria. In D. A. Bryant (Ed.), The Molecular Biology of Cyanobacteria, Advances in Photosynthesis (vol. 1, pp. 559–579). Dordrecht: Kluwer Academic.
Irisarri, P., Gonnet, S., & Monza, J. (2001). Cyanobacteria in Uruguayan rice fields: diversity, nitrogen fixing ability and tolerance to herbicides and combined nitrogen. Journal of Biotechnology, 91, 95–103. doi:10.1016/S0168-1656(01)00334-0.
Jones, C. G., Hare, J. D., & Compaton, S. J. (1989). Measuring plant protein with the Bradford assay: 1. Evaluation and standard method. Journal of Chemical Ecology, 15, 979–992. doi:10.1007/BF01015193.
Kamrin, M. A., & Montgomery, J. H. (2000). Agrochemical and Pesticide Desk Reference on CD-ROM (2nd ed). Boca Raton/New York: Chapman & Hall/CRCnetBASE.
Kidd, H., & James, D. R. (Eds.) (1991). The Agrochemicals Handbook (3rd ed.). Cambridge: Royal Society of Chemistry Information Services.
Kobbia, I. A., Battah, M. G., Shabana, E. F., & Eladel, H. M. (2001). Chlorophyll a fluorescence and photosynthetic activity as tools for the evaluation of simazine toxicity to Protosiphon botryoides and Anabaena variabilis. Ecotoxicology and Environmental Safety, 49, 101–105. doi:10.1006/eesa.2000.1955.
Krieger-Liszkay, A., & Rutherford, A. W. (1998). Influence of herbicide binding on the redox potential of the quinone acceptor in photosystem II: relevance to photodamage and phytotoxicity. Biochemistry, 37, 17339–17344. doi:10.1021/bi9822628.
Leganés, F., & Fernández-Valiente, E. (1992). Effects of phenoxy acetic herbicides on growth, photosynthesis, and nitrogenase activity in cyanobacteria from rice fields. Archives of Environmental Contamination and Toxicology, 22, 130–134. doi:10.1007/BF00213311.
Marker, A. F. H. (1972). The use of acetone and methanol in the estimation of chlorophyll in the presence of phaeophytin. Freshwater Biology, 2, 361–385. doi:10.1111/j.1365-2427.1972.tb00377.x.
Moreland, D. E. (1980). Mechanisms of action of herbicides. Annual Review of Plant Physiology, 31, 597–638. doi:10.1146/annurev.pp.31.060180.003121.
Myklestad, S., & Haug, A. (1972). Production of carbohydrates by the marine diatom Chaetocerus affinis var. willei (Gan) Hustedt. I. Effect of the concentration of nutrients in the culture medium. Journal of Experimental Marine Biology and Ecology, 9, 125–136. doi:10.1016/0022-0981(72)90041-X.
Nicolaus, B., Panico, A., Lama, L., Romano, I., Manca, M. C., De Giulio, A., et al. (1999). Chemical composition and production of exopolysaccharides from representative members of heterocystous and non-heterocystous cyanobacteria. Phytochemistry, 52, 639–647. doi:10.1016/S0031-9422(99)00202-2.
Park, B. J., Kyung, K. S., Choi, J. H., Im, G. J., Kim, I. S., & Shim, J. H. (2005). Environmental fate of the herbicide molinate in a rice-paddy-soil lysimeter. Bulletin of Environmental Contamination and Toxicology, 75, 937–944. doi:10.1007/s00128-005-0840-7.
Quesada, A., Sánchez-Maeso, E., & Fernández-Valiente, E. (1995). Seasonal variation of chemical properties of rice field soils from Valencia, Spain. Communications in Soil Science and Plant Analysis, 26, 1–19.
Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M., & Stanier, R. Y. (1979). Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General Microbiology, 111, 1–61.
Roger, P. A. (1995). The impact of pesticides on ricefield microflora: an analytical review of the literature. In P. L. Pingali, & P. A. Roger (Eds.), Impact of Pesticides on Farmer Health and the Rice Environment (pp. 271–308). Dordrecht: Kluwer Academic.
Roger, P. A., & Kulasooriya, S. A. (1980). Blue-green Algae and Rice. Los Baños/Manila. Philippines: The International Rice Research Institute.
Schagerl, M., & Müller, B. (2006). Acclimation of chlorophyll a and carotenoid levels to different irradiances in four freshwater cyanobacteria. Journal of Plant Physiology, 163, 709–716. doi:10.1016/j.jplph.2005.09.015.
Scherer, S., Almon, H., & Böger, P. (1988). Interaction of photosynthesis, respiration and nitrogen fixation in cyanobacteria. Photosynthesis Research, 15, 95–114. doi:10.1007/BF00035255.
Silva, P. G., & Silva, H. J. (2007). Effect of mineral nutrients on cell growth and self-flocculation of Tolypothrix tenuis for the production of a biofertilizer. Bioresource Technology, 98, 607–611. doi:10.1016/j.biortech.2006.02.026.
Singh, S., & Datta, P. (2006). Screening and selection of most potent diazotrophic cyanobacterial isolate exhibiting natural tolerance to rice field herbicides for exploration as biofertilizer. Journal of Basic Microbiology, 46, 219–225. doi:10.1002/jobm.200510074.
Singh, S., Datta, P., & Patel, R. (2003). Survival and growth of diazotrophic cyanobacterial isolates exposed to rice-field herbicides. Bulletin of Environmental Contamination and Toxicology, 70, 1052–1058. doi:10.1007/s00128-003-0089-y.
Tsimilli-Michael, M., & Strasser, R. J. (2008). In vivo assessment of stress impact on plants’ vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants. In A. Varma (Ed.), Mycorrhiza: State of the art, genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics (pp. 679–703, 3rd ed.). New York: Springer-Verlag.
Tomlin, C. D. S. (Ed.) (2000). The pesticide manual (12th ed.). Berkshire: The British Crop Protection Council Publications.
Vani, B., Mohanty, P., Eggenberg, P., Strasser, B. J., & Strasser, R. J. (1998). Heat stress on rice leaves probed by the fluorescence transient OKJIP. In G. Garab (Ed.), Photosynthesis: Mechanisms and effects: Proceedings of the XIth International Congress on Photosynthesis, Budapest, Hungary 1998 (pp. 3837–3840). Dordrecht: Kluwer Academic.
Vermaas, W. F. J. (2001). Photosynthesis and respiration in cyanobacteria. In: Nature Encyclopedia of Life Sciences. Nature Publishing Group, London [online]. http://www.els.net/ [doi:10.1038/npg.els.0001670], (accessed: January 2008).
Waxman, M. F. (1998). Agrochemical and pesticide safety handbook. Boca Raton: CRC/LLC/Lewis.
Whitton, B. A. (2000). Soils and rice-fields. In B. A. Whitton, & M. Potts (Eds.), The ecology of cyanobacteria: Their diversity in time and space (pp. 233–255). Dordrecht: Kluwer Academic.
Whitton, B. A., & Potts, M. (2000). Introduction to cyanobacteria. In B. A. Whitton, & M. Potts (Eds.), The ecology of cyanobacteria: Their diversity in time and space (pp. 1–11). Dordrecht: Kluwer Academic.
Wyman, M., & Fay, P. (1986). Underwater light climate and the growth and pigmentation of planktonik blue-green algae (cyanobacteria): I. The influence of light quantity. Proceedings of the Royal Society of London. Series B. Biological Sciences, 227, 367–380.
Xia, J. (2005). Response of growth, photosynthesis and photoinhibition of the edible cyanobacterium Nostoc sphaeroides colonies to thiobencarb herbicide. Chemosphere, 59, 561–566. doi:10.1016/j.chemosphere.2004.12.014.
Yan, G. A., Yan, X., & Wu, W. (1997). Effects of the herbicide molinate on mixotrophic growth, photosynthetic pigments, and protein content of Anabaena sphaerica under different light conditions. Ecotoxicology and Environmental Safety, 38, 144–149. doi:10.1006/eesa.1997.1570.
Acknowledgments
This work was supported by a doctoral grant to Victor Galhano from Foundation for Science and Technology, Ministry of Science, Technology and Higher Education, Portuguese Government (ref. SFRH/BD/17582/2004). The authors are indebted to the anonymous referee for a critical and constructive reading of the manuscript and also for valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galhano, V., Peixoto, F., Gomes-Laranjo, J. et al. Differential Effects of Bentazon and Molinate on Anabaena cylindrica, an Autochthonous Cyanobacterium of Portuguese Rice Field Agro-ecosystems. Water Air Soil Pollut 197, 211–222 (2009). https://doi.org/10.1007/s11270-008-9804-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9804-y