Abstract
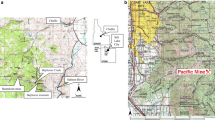
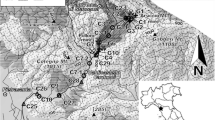
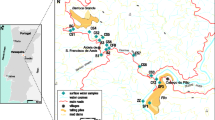
Former mining activities lasting 140 years in the Ducktown Mining District, Tennessee, USA, has contaminated the streams draining the district with acid-mine drainage (AMD). North Potato Creek and its major tributary, Burra Burra Creek, are two of the most heavily AMD-impacted streams in the district. The removal of dissolved metals from the water in these creeks is largely attributable to the sorption of Cu, Zn, Co, Al, and Mn on suspended hydroxide precipitates of Fe. The fraction of trace metals remaining in solution decreases with increasing pH in the sequence Pb < Cu < Zn < Co. The concentration of Fe in solution also decreases with increasing pH due to the formation of ferric hydroxide precipitates which accounted for up to 81.4% by weight of the total suspended sediment. The concentration of suspended sediment substantially decreases as the water of North Potato Creek flows through a large settling basin, where 1.3 (±0.3) × 106 kg/year of trace-metal-laden suspended sediment would be annually deposited. In spite of this attempt to purify it, the water discharged into the river is acidic (pH 3.6) and still contains high concentrations of dissolved trace metals, which would resorb on to suspended sediment and be ultimately transported to a downstream reservoir, Ocoee No. 3 Lake.







Similar content being viewed by others
References
Alexander, F. M., Keck, L. A., Conn, L. G., & Wentz, S. J. (1984). Drought-related impacts on municipal and major self-supplied industrial water withdrawals in Tennessee, Part B. USGS Water Resources Investigation Report, 84-4074, 398.
Benjamin, M. M., & Leckie, J. O. (1981). Multiple-site adsorption of Cd, Cu, Zn and Pb on amorphous iron oxyhydroxide. Journal of Colloid and Interface Science, 79, 209–221.
Bigham, J. M., Schwertmann, U., & Pfab, G. (1996a). Influence of pH on mineral speciation in a bioreactor simulating acid mine drainage. Applied Geochemistry, 11, 845–849.
Bigham, J. M., Schwertmann, U., Traina, S. J., Winlnd, R. L., & Wolf, M. (1996b). Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochimica et Cosmochimica Acta, 60, 2111–2121.
Brunke, M. (1999). Colmation and depth filtration within streambeds: retention of particles in hyporheic interstices. International Review of Hydrobiology, 84, 99–117.
Chapman, B. M., Jones, D. R., & Jung, R. F. (1983). Processes controlling metal ion attenuation in acid mine drainage streams. Geochimica et Cosmochimica Acta, 47, 1957–1973.
Dzombak, D. A., & Morel, F. M. M. (1990). Surface complexation modeling: Hydrous ferric oxide. New York: Wiley.
Hammarstrom, J. M., Seal, R. R., Meier A. L., & Kornfeld, J. M. (2005). Secondary sulfate minerals associated with acid drainage in the eastern US: recycling of metals and acidity in surficial environments. Chemical Geology, 215(1–4), 407–431.
Jambor, J. L. (1994). Mineralogy of sulfide-rich tailings and their oxidation products. In: J. L. Jambor & D. W. Blowes (Eds.), Short course handbook on environmental geochemistry of sulfide-mine-wastes (pp. 59–102). Ontario: Waterloo.
Jenne, E. A. (1968). Controls on Mn, Fe, Co, Ni, Cu, and Zn concentrations in soils and water: the significant role of hydrous Mn and Fe oxides. Advances in Chemistry Series, 73, 337–387.
Johnson, C. A. (1986). The regulation of trace element concentrations in river and estuarine waters contaminated with acid mine drainage: The adsorption of Cu and Zn on amorphous Fe oxyhydroxides. Geochimica et Cosmochimica Acta, 50, 2433–2438.
Karlsson, S., Allard, B., & Häkansson, K. (1988a). Chemical characterization of stream-bed sediments receiving high loadings of acid mine effluents. Chemical Geology, 67, 1–15.
Karlsson, S., Allard, B., & Häkansson, K. (1988b). Characterization of suspended solids in a stream receiving acid mine effluents, Bersbo, Sweden. Applied Geochemistry, 3, 345–356.
Kimball, B. A., Broshears, R. E., McKnight, D. M., & Bencala, K. E. (1994). Effects of instream pH modification on transport of sulfide-oxidation products. In: C. N. Alpers & D. W. Blowes (Eds.), Environmental geochemistry of sulfide oxidation. ACS symposium series 550 (pp. 224–243). Washington, DC: American Chemical Society.
Kinniburgh, D. G., Jackson, M. L., & Syers, J. K. (1976). Adsroption of alkaline earth, transition, and heavy metal cations by hydrous oxide gels of iron and aluminum. Soil Science Society of American Journal, 40, 796–799.
Laxen, D. P. (1985). Trace metal adsorption/coprecipitation on hydrous ferric oxide under realistic conditions. Water Research, 19, 1229–1236.
Lee, G. (2001). Transport and fate of trace metals in streams contaminated with acid mine-drainage in the Ducktown Mining District, Tennessee. Dissertation, Ohio State University.
Lee, G., Bigham, J. M., & Faure, G. (2002). Removal of trace metals by coprecipitation with Fe, Al, and Mn from natural waters contaminated by acid mine drainage in Ducktown Mining District, Tennessee. Applied Geochemistry, 17, 569–581.
Nordstrom, D. K., & Alpers, C. N. (1999). Geochemistry of acid mine waters. In: G. S. Plumlee & M. J. Logsdon (Eds.), The Environmental Geochemistry of Mineral Deposits, Rev. Econ. Geol. vol. 6A. (pp. 133–160) Littleton: Society of Economic Geologists, Inc.
Packman, A. I., & MacKay, J. S. (2003). Interplay of stream-subsurface exchange, clay particle deposition, and stream bed evolution. Water Resources Research, 39, 1097.
Parkhurst, D. L. (1995). Users guide to PHREEQC—a computer program for speciation, reaction-path, advective transport, and inverse geochemical calculations. USGS Water Resources Investigation Report, 95-4227, 143.
Paulson, R. W., Chase, E. B., Williams, J. S., & Moody, D. W. (1993). National water summary 1990–91. Hydrologic events and stream water quality. USGS Water Supply Paper, 2400, 499–506.
Piatak, N. M., Seal, R. R., & Hammarstrom, J. M. (2004). Mineralogical and geochemical controls on the release of trace elements from slag produced by base- and precious-metal smelting at abandoned mine sites. Applied Geochemistry, 19(7), 1039–1064.
Rampe, J. J., & Runnells, D. D. (1989). Contamination of water and sediment in a desert stream by metals from an abandoned gold mine and mill, Eureka District, Arizona, U.S.A. Applied Geochemistry, 4, 445–454.
Rehg, K. J., Packman, A. I., & Ren, J. (2005). Effects of suspended sediment characteristics and bed sediment transport on streambed clogging. Hydrological Processes, 19, 413–427.
Stumm, W., & Morgan, J.J. (1996). Aquatic chemistry: Chemical equilibria and rates in natural waters (3rd ed.). New York: Wiley.
Sullivan, P. J., Yelton, J. L, & Reddy, K. J. (1988). Iron sulfide oxidation and the chemistry of acid generation. Environmental Geology and Water Science, 11, 289–295.
Theobald, P. K. Jr., Lakin, H. W., & Hawkins, D. B. (1963). The precipitation of aluminum, iron and manganese at the junction of Deer Creek with the Snake River in Summit County, Colorado. Geochimica et Cosomochimica Acta, 27(2), 121–132.
U.S. Environmental Protection Agency (2005). Copper Basin Mining District case study: Use of cooperative agreements. http://www.epa.gov/superfund/programs/aml/tech/copperbasin.pdf.
U. S. Geological Survey (1973). Surface water supply of the United States, 1966–70, part 3. Ohio River Basin below Wabash River. USGS Water Supply Paper 2110.
Walling, D. E., & Webb, B. W. (1996). Water quality; physical characteristics. In: G. Petts & P. Calow (Eds.), River flows and channel forms (pp. 77–101). Oxford: Blackwell Science.
Wood, P. J., & Armitage, P. D. (1997). Biological effects of fine sediment in the lotic environment. Environmental Management, 21, 203–217.
Acknowledgments
The authors express gratitude for valuable discussions to Jerry M. Bigham on the characterization of suspended sediment and to Douglas E. Pride on the history and the mines of the Ducktown Mining District. This work was supported by the Graduate Student Alumni Research Award of the Graduate School of The Ohio State University and the Korean Research Foundation (KRF-2005-070-C00137).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, G., Faure, G. Processes Controlling Trace-Metal Transport in Surface Water Contaminated by Acid-Mine Drainage in the Ducktown Mining District, Tennessee. Water Air Soil Pollut 186, 221–232 (2007). https://doi.org/10.1007/s11270-007-9479-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9479-9