Abstract
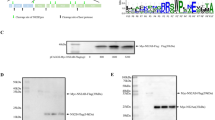
Rana grylio virus (RGV), a member of genus Ranavirus in the family Iridoviridae, is a viral pathogen infecting aquatic animal. RGV 43R has homologues only in Ranavirus and contains a transmembrane (TM) domain, but its role in RGV infection is unknown. In this study, 43R was determined to be associated with virion membrane. The transcripts encoding 43R and the protein itself appeared late in RGV-infected EPC cells and its expression was blocked by viral DNA replication inhibitor, indicating that 43R is a late expressed protein. Subcellular localization showed that 43R-EGFP fusion protein distributed in cytoplasm of EPC cells and that TM domain is essential for its distribution in cytoplasm. 43R-EGFP fusion protein colocalized with viral factories in RGV-infected cells. A recombinant RGV deleting 43R (Δ43R-RGV) was constructed by homologous recombination to investigate its role in virus infection. Compared with wild type RGV, the ability of Δ43R-RGV to induce the cytopathic effect and its virus titers were significantly reduced. Furthermore, it is revealed that 43R deletion significantly inhibited viral entry but did not influence viral DNA replication by measuring and comparing the DNA levels of RGV and Δ43R-RGV in the infected cells at the early stage of infection. RGV neutralization with anti-43R serum reduced the virus titer. Therefore, these data showed that RGV 43R is a late gene that encodes an envelope protein involved in RGV entry.








Similar content being viewed by others
References
Zhang QY, Gui JF (2012) Atlas of aquatic viruses and viral diseases. Science Press, Beijing
Zhang QY, Gui JF (2015) Virus genomes and virus-host interactions in aquaculture animals. Sci China Life Sci 58(2):156–169
Jancovich JK, Bremont M, Touchman JW, Jacobs BL (2010) Evidence for multiple recent host species shifts among the ranaviruses (family Iridoviridae). J Virol 84(6):2636–2647
Chinchar VG, Waltzek TB, Subramaniam K (2017) Ranaviruses and other members of the family Iridoviridae: their place in the virosphere. Virology 511:259–271
Lucas W, Knipe DW (2010) Viral capsids and envelopes: structure and function. Macmillan Publishers Ltd, London
Robert J, Jancovich JK (2016) Recombinant ranaviruses for studying evolution of host-pathogen interactions in ectothermic vertebrates. Viruses 8(7):187
Yuan YM, Wang YZ, Liu QZ, Zhu F, Hong YH (2016) Singapore grouper iridovirus protein VP088 is essential for viral infectivity. Sci Rep 6:31170
Kim YS, Ke F, Lei XY, Zhu R, Zhang QY (2010) Viral envelope protein 53R gene highly specific silencing and iridovirus resistance in fish cells by AmiRNA. PLoS ONE 5(4):e10308
He LB, Gao XC, Ke F, Zhang QY (2013) A conditional lethal mutation in Rana grylio virus ORF 53R resulted in a marked reduction in virion formation. Virus Res 177(2):194–200
He LB, Ke F, Wang J, Gao XC, Zhang QY (2014) Rana grylio virus (RGV) envelope protein 2L: subcellular localization and essential roles in virus infectivity revealed by conditional lethal mutant. J Gen Virol 95(3):679–690
Lei XY, Ou T, Zhu RL, Zhang QY (2012) Sequencing and analysis of the complete genome of Rana grylio virus (RGV). Arch Virol 157(8):1559–1564
Eaton HE, Metcalf J, Penny E, Tcherepanov V, Upton C, Brunetti CR (2007) Comparative genomic analysis of the family Iridoviridae: re-annotating and defining the core set of iridovirus genes. Virol J 4(1):11
Zhao Z, Ke F, Gui JF, Zhang QY (2007) Characterization of an early gene encoding for dUTPase in Rana grylio virus. Virus Res 123(2):128–137
Ke F, Zhao Z, Zhang QY (2009) Cloning, expression and subcellular distribution of a Rana grylio virus late gene encoding ERV1 homologue. Mol Biol Rep 36(7):1651–1659
Lei XY, Ou T, Zhang QY (2012) Rana grylio virus (RGV) 50L is associated with viral matrix and exhibited two distribution patterns. PLoS ONE 7(8):e43033
Ke F, Gui JF, Chen ZY, Li T, Lei CK, Wang ZH, Zhang QY (2018) Divergent transcriptomic responses underlying the ranaviruses-amphibian interaction processes on interspecies infection of Chinese giant salamander. BMC Genomics 19(1):211
Nicholas KB, Nicholas HBJ, Deerfield DW (1997) GeneDoc: analysis and visualization of genetic variation. Embnew News 4:14
He LB, Ke F, Zhang QY (2012) Rana grylio virus as a vector for foreign gene expression in fish cells. Virus Res 163(1):66–73
Wang J, Gui L, Chen ZY, Zhang QY (2016) Mutations in the C-terminal region affect subcellular localization of crucian carp herpesvirus (CaHV) GPCR. Virus Genes 52(4):484–494
Chen ZY, Li T, Gao XC, Wang CF, Zhang QY (2018) Protective immunity induced by DNA vaccination against ranavirus infection in Chinese giant salamander Andrias davidianus. Viruses 10(2):52
Wan QJ, Gong J, Huang XH, Huang YH, Zhou S, Ou-Yang ZL, Cao JH, Ye LL, Qin QW (2010) Identification and characterization of a novel capsid protein encoded by Singapore grouper iridovirus ORF038L. Arch Virol 155(3):351–359
Wang Q, Luo YW, Xie JF, Dong CF, Weng SP, Ai HS, Lü L, Yang XQ, Yu XQ, He JG (2008) Identification of two novel membrane proteins from the tiger frog virus (TFV). Virus Res 136(1–2):35–42
Zhou S, Wan QJ, Huang YH, Huang XH, Cao JH, Ye LL, Lim TK, Lin QS, Qin QW (2011) Proteomic analysis of Singapore grouper iridovirus envelope proteins and characterization of a novel envelope protein VP088. Proteomics 11(11): 2236–2248
Ouyang ZL (2010) Screening of DNA vaccine against Singapore grouper iridovirus (SGIV) and characterization of an envelope protein VP16. Dissertation, Sun Yat-sen University
Majji S, Thodima V, Sample R, Whitley D, Deng Y, Mao J, Chinchar VG (2009) Transcriptome analysis of Frog virus 3, the type species of the genus Ranavirus, family Iridoviridae. Virology 391(2):293–303
Guo HT, Shen S, Wang LL, Deng HY (2010) Role of tegument proteins in herpesvirus assembly and egress. Protein Cell 1(11):987–998
Pitts JD, Klabis J, Richards AL, Smith GA, Heldwein EE (2014) Crystal structure of the herpesvirus inner tegument protein UL37 supports its essential role in control of viral trafficking. J Virol 88(10):5462–5473
De Beeck AO, Rouillé Y, Caron M, Duvet S, Dubuisson J (2004) The transmembrane domains of the prM and E proteins of yellow fever virus are endoplasmic reticulum localization signals. J Virol 78(22):12591–12602
Zhao Z, Ke F, Huang YH, Zhao JG, Gui JF, Zhang QY (2008) Identification and characterization of a novel envelope protein in Rana grylio virus. J Gen Virol 89(8):1866–1872
Zhang HL, Zhou S, Xia LQ, Huang XH, Huang YH, Cao JH, Qin QW (2015) Characterization of the VP39 envelope protein from Singapore grouper iridovirus. Can J Microbiol 61(12):924–937
Huang XH, Huang YH, Yuan XP, Zhang QY (2006) Electron microscopic examination of the viromatrix of Rana grylio virus in a fish cell line. J Virol Methods 133(2):117–123
Jancovich JK, Qin QW, Zhang QY, Chinchar VG (2015) Ranavirus replication: molecular, cellular, and immunological events. In: Gray MJ, Chinchar VG (eds) Ranaviruses: lethal pathogens of ectothermic vertebrates. Springer, Cham, pp 105–139
Guo CJ, Wu YY, Yang LS, Yang XB, He J, Mi S, Jia KT, Weng SP, Yu XQ, He JG (2012) Infectious spleen and kidney necrosis virus (a fish iridovirus) enters mandarin fish fry cells via caveola-dependent endocytosis. J Virol 86(5):2621–2631
Wang SW, Huang XH, Huang YH, Hao X, Xu HJ, Cai MJ, Wang HD, Qin QW (2014) Entry of a novel marine DNA virus, Singapore grouper iridovirus, into host cells occurs via clathrin-mediated endocytosis and macropinocytosis in a pH-dependent manner. J Virol 88(22):13047–13063
Acknowledgements
This work is supported by grants from the National Natural Science Foundation of China (31430091, 31772890).
Author information
Authors and Affiliations
Contributions
QYZ conceived and designed the study. XCG and XTZ performed experiments. QYZ and XTZ analyzed the data and wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Additional information
Edited by Zhen F. Fu.
Rights and permissions
About this article
Cite this article
Zeng, XT., Gao, XC. & Zhang, QY. Rana grylio virus 43R encodes an envelope protein involved in virus entry. Virus Genes 54, 779–791 (2018). https://doi.org/10.1007/s11262-018-1606-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-018-1606-8