Abstract
The NS3 protein is a multifunctional non-structural protein of flaviviruses implicated in the polyprotein processing. The predominance of cytotoxic T cell lymphocytes epitopes on the NS3 protein suggests a protective role of this protein in limiting virus replication. In this work, we studied the antigenicity and immunogenicity of a recombinant NS3 protein of the Dengue virus 2. The full-length NS3 gene was cloned and expressed as a His-tagged fusion protein in Escherichia coli. The pNS3 protein was purified by two chromatography steps. The recombinant NS3 protein was recognized by anti-protease NS3 polyclonal antibody and anti-DENV2 HMAF by Western Blot. This purified protein was able to stimulate the secretion of high levels of gamma interferon and low levels of interleukin-10 and tumor necrosis factor-α in mice splenocytes, suggesting a predominantly Th-1-type T cell response. Immunized BALB/c mice with the purified NS3 protein showed a strong induction of anti-NS3 IgG antibodies, essentially IgG2b, as determined by ELISA. Immunized mice sera with recombinant NS3 protein showed specific recognition of native dengue protein by Western blotting and immunofluorescence techniques. The successfully purified recombinant protein was able to preserv the structural and antigenic determinants of the native dengue protein. The antigenicity shown by the recombinant NS3 protein suggests its possible inclusion into future DENV vaccine preparations.
Similar content being viewed by others
Introduction
Dengue virus (DENV) infection has become a growing threat to public health in the tropical regions of the world during the last decades. Annually, an estimated of 50–100 million cases of dengue fever (DF) and several hundred thousand cases of dengue hemorrhagic fever (DHF) occur, depending on epidemic activity [1]. Four antigenically distinct serotypes (dengue 1–4), members of the family Flaviviridae, are transmitted to humans by the bite of a domestic mosquito, Aedes aegypti [2]. They are responsible for a spectrum of dengue diseases ranging from asymptomatic infection, passing through mild fever illness, DF, to more severe forms DHF and dengue shock syndrome (DSS) [3–5]. Although primary infection with dengue viruses is thought to induce lifelong protective immunity to the infecting serotype, it does not confer long-term cross protective immunity against the other viruses. Hence, an ideal dengue vaccine must be tetravalent, capable of conferring protection against infection by all four serotypes, or should prevent disease due to dengue infection [6]. Several studies have found a positive correlation between the presence of virus-neutralizing antibody and protection [7–10]. However, other factors more than neutralizing antibody play a crucial role in protection. The activation of T cells as a cellular immune response could protect against DENV in the absence of neutralizing antibody [11–15]. In this sense, a vaccine approach based on inducing DENV-specific cytotoxic T lymphocytes (CTL) and an appropriate humoral immunity should provide the best balance of protective dengue immune response [16]. Some DENV proteins stimulate immunity mediated by CTL among them the NS3 protein have been identified as the major source of serotype-specific and serotype-cross-reactive CD4+ and CD8+ CTL epitopes [17–21]. These CTL may play a protective role in limiting virus replication [22, 23]. The NS3 protein, the second largest DENV non-structural protein, mediates the activities associated with virus replication, including protease, NTPase, helicase, and RNA binding and also capping of the viral genomic RNA [24–27]. In spite of the structure and function of the NS3 protein has been extensively studied, few reports have been undertaken in order to evaluate the antigenicity and immunogenicity of the NS3 protein from DENV, as well as, other Flavivirus.
In the present work, the immunogenicity of the full-length DENV2-NS3 protein (DENV2-NS3) produced in Escherichia coli (E. coli) and highly purified was evaluated in BALB/c mice. The antigenic reactivity of the recombinant NS3 protein against a panel of murine antibodies from all four serotypes was determined, as well as, by estimating the cytokine secretion profile from DENV2-infected mouse splenocytes stimulated in vitro by the recombinant NS3. We demonstrated that the purified NS3 protein preserved the antigenic integrity of the viral native protein and elicited high anti-recombinant antibody titers.
Materials and methods
Bacterial strains, cells, antibodies, and viral antigens
Escherichia coli XL1-blue strain was used for the propagation of plasmids and protein expression.
An anti-Histidine monoclonal antibody, MAb, (Qiagen, USA,), anti-NS3 protease polyclonal antibody, and dengue 2- specific mouse monoclonal antibody 3H5-1-21 were used for immunodetection assay.
Aedes albopictus cell line (C6/36 HT) [28] was kindly provided by Dr. Javier Diaz from laboratory of Medellín department, Colombia. C636 cells were grown at 33 °C in Eagle’s minimal essential medium supplemented with 10 % heat-inactivated fetal bovine serum (HFBS), 1 % non-essential amino acids, and 1 % glutamine solution (200 mM). African green monkey kidney (Vero) cells (ATCC, CCL-81) were grown in 199 medium (Gibco, USA) supplemented with 10 % HFBS (SIGMA, USA). These cells were maintained in the same medium with 2 % of HFBS.
Pools of polyclonal hyperimmune mouse ascitic fluids (HMAFs) to the four DENV, used for the antigenic characterization of the recombinant protein were obtained from reference strains as described Brandt et al. 1967 [29]. Briefly, mice were immunized by four intraperitoneal injections per week of 10 % crude suspensions of infected suckling mouse brain in phosphate-buffered saline (PBS) mixed with Freund’s adjuvant. To induce ascites formation, sarcoma 180 cells were given intraperitoneally with the final injection. The ascites fluids were obtained by peritonea puncture, and then centrifuged at 1,000×g for 30 min at 4 °C. The supernatant was collected and stored at −80 °C.
A preparation from suckling mice brain infected with DENV2, A-15 strain, Cuba [30] was used as antigen (106 p.f.u.) for antibody detection and mice immunization [31]. A similar preparation obtained from brain of non-inoculated mice was used as negative control (Mock).
A concentrated preparation of DENV2 antigen was used for Western blotting (WB), indirect immunofluorescence assay (IFA), and in vitro stimulation of mouse splenocytes. Briefly, supernatant from Vero cells infected with 106 p.f.u/mL of DENV2 SB8553 strain, kindly provided by M.J. Cardosa, University Sarawak, Malaysia was concentrated by centrifugation at 80,000×g, 4 h at 4 °C. The pellet containing the virus was resuspended in PBS (Gibco, UK). A mock preparation was similarly prepared from the supernatant of non-infected Vero cells.
Construction of 6His-NS3 expression plasmid
The full-length DENV2 NS3 gene (1.8 Kb) was obtained by reverse transcriptase-polymerase chain reaction (RT-PCR) amplification of the RNA extracted from a culture supernatant of DENV2 58/97 2 passages in C6/36 HT (strain from Cuban patient with DHF during a 1997 outbreak in Santiago de Cuba). Primers used for the RT-PCR were: forward 5′-ctgcagCGGGCTGGAGTA-3′ and reverse 5′-aagcttTTATTTTCTTCCAGCTGCAAATTCC-3′ containing PstI and HindIII restriction sites, respectively. Briefly, this technique was performed under the following conditions: 30 cycles of 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 2 min using GoTaq DNA polymerase (Promega, USA), at last 10 min at 72 °C to complete the PCR reaction. The resulting PCR product (DENV2-NS3) was directly cloned into a pGEM-T Easy vector (Promega) for sequence verification and further procedures. The DENV2-NS3 gene fragment was then subcloned into an expression vector pQE-30 (Qiagen) at the PstI and HindIII sites downstream of N-terminal 6 × His tag, generating the recombinant plasmid, pQE30NS3.
NS3 expression and purification
To express the recombinant NS3 protein, the E. coli strain XL1-blue was electrotransformed with pQE30NS3 plasmids following instructions from QiaExpressionist manual (Qiagen). A single transformed colony harboring pQE30NS3 was grown overnight at 37 °C in Luria-Bertania (LB) medium containing 50 µg/mL of ampicillin. Overnight cultures were 100-fold diluted in the LB medium with antibiotics and further incubated at 37 °C until the optical density at 600 nm reached 0.5. DENV2-NS3 expression was then induced by the addition of 1 mM of isopropylthiogalactoside (IPTG) (Sigma-Aldrich, USA) for 4 h at 37 °C. To analyze the expression of NS3 protein, samples were centrifuged at 10,000×g for 10 min at 4 °C. The bacterial pellet was then analyzed by electrophoresis. Similar expression procedure was made to XL1-blue cells transformed with the pQE-30 plasmid (E. coli negative preparation).
For the purification process, cells were harvested and resuspended in buffer A (50 mM Tris–HCl, pH 8, 5 mM β-mercaptoethanol (βMeOH), 500 mM NaCl, and 1 mM PMSF). The cellular suspension was immediately disrupted by freezing and defrosting cycles in liquid N2 and then centrifuged at 15,000×g for 20 min at 4 °C to separate the soluble protein fraction and pellet (insoluble protein fraction) for further analysis. The pellet fraction containing inclusion bodies was resuspended in buffer B (8 M urea, 50 mM Tris–HCl, pH 8, 5 mM βMeOH, 200 mM NaCl, and 1 mM PMSF) and incubated overnight at 4 °C; the lysate was then clarified by centrifugation at 15,000×g for 1 h at 4 °C. The solubilized protein fraction was loaded onto a HisTrap™ affinity column (GE Healthcare Life Sciences, USA) previously equilibrated with buffer B, using HPLC- AKTA purifier system (Amersham Biosciences, USA). The bound proteins were washed with buffer C (buffer B pH 6.3). Proteins were eluted with a linear gradient using buffer D (8 M urea, 50 mM Tris–HCl, pH 8, 5 mM βMeOH, 200 mM NaCl, 1 mM PMSF, and 500 mM imidazole). Peak fractions (3 mL) were pooled and diluted until an absorbance at 280 nm between 0.8 and 1.0. Denatured NS3 protein was refolded by dialysis in buffer E (50 mM Tris–HCl, pH 8, 500 mM NaCl, 10 % glycerol, 5 mM βMeOH, and 0.05 % CHAPS) containing urea at different concentrations, decreasing in a stepwise manner (6, 4, 2, 1, and 0.01 M urea every 1 h, and no urea overnight) at 4 °C. The dialysate product was concentrated by ultrafiltration using YM-30 membrane (Amicon, USA), and then applied to a HiPrep 26/60 Sephacryl S-100 HR gel filtration column (GE Healthcare Life Sciences) using a flow of 1.0 mL/min. The peak fractions were pooled and concentrated to 5 mg/mL. All protein samples were analyzed by electrophoresis. A protein preparation from disrupted XL1-blue/pQE30 cells was purified by affinity chromatography and it was considered as a purified E. coli negative preparation for all purposes.
Protein analysis
Protein samples were subjected to 12 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as described earlier [32]. Protein bands were visualized by staining with Coomassie brilliant blue R-250 (Sigma). The protein concentration was measured using Bradford assay kit (Bio-Rad Laboratories, USA) [33].
Immunoassays for recombinant NS3 protein detection
WB
Protein samples were electrotransferred from the polyacrylamide gel to a nitrocellulose membrane as described by [34]. The membrane was blocked overnight by incubation with 4 % skimmed milk in PBS-T (0.8 % NaCl, 0.02 % KCl, 0.014 % KH2PO4, 0.009 % Na2HPO4, pH 7.4, and 0.05 % Tween-20) at 4 °C, washed three times with PBS-T, and then incubated 2 h at room temperature (RT) with the primary antibody anti-DENV2 HMAF at 1:100 dilution in 4 % skimmed milk in PBS-T. Once washed, the membrane was reacted with goat anti-mouse IgG peroxidase conjugate (GE Healthcare Life Sciences) at 1:2,000 dilution in PBS-Tween for 1 h at RT. Afterwards, the membrane was washed again and the bands were detected by incubation, using a diaminobenzidine (DAB) chromogenic substrate solution until the color development was completed. NS3 detection was also evaluated with anti-NS3 protease polyclonal antibody (dilution 1:1,000) and anti-His MAb (dilution 1:1,000) using ECL plus WB detection system (GE Healthcare Life Sciences).
ELISA
To measure the reactivity of the recombinant protein DENV2-NS3 protein against HMAFs corresponding to the four DENV serotypes, an indirect ELISA system was performed. Polystyrene plates of 96 wells (Greiner bio-one, Germany) were coated for 2 h at 37 °C with 5 µg/mL NS3 recombinant protein and as positive control, 100 µL of DENV2 antigen diluted in coating buffer (0.16 % Na2CO3, 0.29 % NaHCO3, and pH 9.5). As negative control plates were coated with 5 μg/mL of the purified E. coli negative preparation and 100 μL of Mock. Subsequently, plates were blocked with 2 % bovine serum albumin (BSA) for 1 h at 37 °C in the same buffer solution. Three washes with PBS-T were completed after each step of ELISA. Once washed, HMAFs of each viral serotype were serially diluted and incubated for 1 h at 37 °C. Goat anti-mouse IgG peroxidase conjugate at 1:2,500 dilution was added and plates were again incubated for 1 h at 37 °C. After washing, the reaction was developed with 100 μL of 500 μg/mL ortho-phenylenediamine dihydrochloride (OPD) as chromogen and 0.015 % hydrogen peroxide as substrate in 0.1 M citrate buffer (2 % Na2HPO4, 1 % citric acid, pH 5.0). Plates were kept for 30 min at 25 °C and the reaction was stopped with 12.5 % H2SO4. The absorbance was measured at 490 nm using an ELISA plate reader (Bio-Rad Laboratories). Mean values and standard errors of the mean for each group were calculated. The positive cut-off value was taken as twice the absorbance value of the negative control.
Cell culture and viral stimulation
Spleen cells of mice immunized with DENV2 were obtained in aseptic conditions, and erythrocytes were lysed by adding 0.83 % NH4Cl solution. Cells from each animal were washed twice with PBS and 2 % HFBS (PAA Laboratories, Ontario, Canada) and resuspended at 2 × 106 cells/mL in RPMI-1640 medium supplemented with 100 U/mL penicillin, 100 g/mL streptomycin (Gibco, USA), 2 mM glutamine (Gibco), 5 × 105 M βMeOH (Sigma), and 5 % HFBS. Finally, 2 × 105 cells per well were cultured in 96-well round bottom plates with the antigens (moi 1 of DENV2 antigen or Mock preparation and 10 µg/mL purified NS3 protein). ConA (Sigma) was used as a positive control. After 4 days of 5 % CO2 incubation at 37 °C, culture supernatants were collected and stored at −20 °C. All assays were run in triplicate.
Cytokine detection
The culture supernatants of splenocytes stimulated with the recombinant NS3 protein and DENV2 antigen were analyzed in duplicate to determine the gamma interferon (IFN-γ), interleukin-10 (IL-10), and tumor necrosis factor (TNF-α) concentration by ELISA using MAbs pairs (BD Biosciences Pharmingen, USA). ELISA protocol recommended by manufacturers was used with slight modifications and the values were expressed as mean ± standard deviation (SD). The lower limit of detection of cytokine was 4 pg/mL.
Mice immunization
Three groups of 7 female 3-week-old BALB/c mice (CENPALAB, Cuba) were immunized by intraperitoneal (i.p) route on days 0, 14, and 28 with 20 µg of the purified recombinant NS3 protein per dose, emulsified (v/v) with 0.05 mL of Freund adjuvant (complete for the first dose and incomplete for the others). Similarly, a group of mice received 20 µg of the purified E. coli negative preparation, negative control. As a positive control, a mice group was inoculated by i.p route with one dose of 106 p.f.u of DENV2 without adjuvant. Mice were bled 15 days after the last dose and sera were collected for further immunological analysis. The protocols for the maintenance and care of experimental animals were approved by the IPK-Ethics Committee.
Antibody titer against recombinant protein
The anti-NS3 antibody level in the serum of immunized mice was determined by an indirect ELISA. Briefly, polystyrene plates of 96 wells were coated with 100 μL of the recombinant protein in coating buffer and incubated 2 h at 37 °C. Plates were blocked with 100 μL of 1 % BSA in coating buffer for 2 h at 37 °C and then washed three times in PBS-T. Mice sera serially diluted twofold in PBS-T starting with 1:100 were incubated in triplicate wells (100 μL/well) overnight at 4 °C. Goat anti-mouse IgG peroxidase conjugate diluted 1:25,000 was added, and plates were incubated for 1 h at 37 °C. Finally, the reaction signal was revealed with OPD. 100 μL of the purified E. coli negative preparation was used as negative control. Dilutions of sera at which the absorbance value is twice the value of the negative control were considered to be the ELISA end point titers.
Antibody isotyping
Polystyrene plates were coated overnight at 4 °C with DENV2-NS3 (5 μg/mL) in coating buffer. Plates were washed with PBS-T and blocked with 5 % BSA (100 μL/well) for 2 h at 37 °C. After blocking, the plates were washed with PBS-T and sera were added at dilutions of 1:1,000 to 1:32,000 in PBS-T and incubated for 1 h, then the plates were washed and incubated with goat anti-mouse IgG isotype-specific antibodies IgG1, IgG2a, IgG2b, and IgG3 (Sigma) diluted 1:1,000 for 30 min at 37 °C. After washing, plates were incubated with anti-goat HRP-conjugated antibody diluted 1:5,000 for 15 min at 37 °C and washed as mentioned above. The color was developed with 100 μL of OPD and hydrogen peroxide. The reaction was stopped by adding 12.5 % sulfuric acid. Absorbance was measured on an ELISA reader at 490 nm.
Immunodetection of native NS3 protein
WB
supernatants from C6/36 HT cells infected with DENV-2 were mixed with the sample buffer (without βMeOH) for SDS-PAGE and heated for 5 min at 80 °C as previously described [31]. The mixture was loaded onto a 10 % polyacrylamide gel; electrophoresis and blotting were assessed [32, 34]. After blocking with 5 % skim milk for 1 h at room temperature, the nitrocellulose membrane was cut into strips and incubated overnight at 4 °C with 1:30-diluted mice sera. As positive control, DENV proteins were identified by incubating one strip with DENV2 HMAF at 1:100. A pool of mice sera from negative control group and NS3-immunized group were individually tested. Finally, the strips were incubated with a 1:1,000 dilution of goat anti-mouse IgG peroxidase conjugate for 1 h at 37 °C. The reaction was detected by using DAB solution until the color development.
IFA
C6/36 HT cells maintained at 37 °C in CO2 incubator in Dulbeco’s modified eagle’s medium (DMEM) were infected with DENV2. After 48-h infection, cells were collected and evaluated by IFA [35]. Mice sera of NS3-inoculated and negative control groups at a dilution 1:30 were used to detect the presence of viral antigen in inoculated cells. In this assay, non-inoculated cells (negative control) were run in parallel as DENV2-inoculated C6/36 cells (positive control) and evaluated using dengue 2- specific mouse monoclonal antibody 3H5-1-21 (Pan-American Health Organization). The antigen–antibody interaction was detected by an anti-mouse IgG FITC conjugate (Sigma-Aldrich, USA). The intensity of the FITC fluorochrome dyes was observed by microscopy (Leika 40×) at Ex/Em = 490/520 nm.
Statistical analysis
Data from the ELISA were processed by the GraphPad Prism program (Version 4, 2003), using a parametric statistical analysis of multiple comparison (Newman–Keuls) or Kruskal–Wallis non-parametric test with Dunn’s corrections in relation to the analysis of normality and variance homogeneity.
Results
Cloning, expression, and purification of the recombinant DENV2-NS3 protein
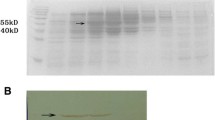
An amplified fragment of the viral genome corresponding to full-length of DENV2NS3 protein was successfully cloned into the pQE-30 expression vector containing the 6xHis-tag. The cloned sequence was 99 % identity to DENV2 New Guinea “C” strain retrieved from GenBank AF038403. XL1-blue cells transformed with the plasmid pQE30NS3 expressed a major band of ~70 kDa detected by SDS-PAGE (Fig. 1a). After cell disruption, the supernatant did not show any band corresponding to the recombinant protein, indicating that NS3 was predominantly associated with the insoluble fraction, as E. coli inclusion bodies. The recombinant DENV2-NS3 protein was immunoidentified by WB with an anti-DENV2 HMAF (Fig. 1b). To avoid aggregation of the NS3 protein, the purification process was carried out under denaturing conditions, so that the insoluble fraction was completely dissolved in 8 M urea before chromatographic steps. After affinity chromatography, the recombinant protein reached 60 % purity and the purified fraction was associated with detectable amounts of low-size contaminants (Fig. 2; Table 1). SDS-PAGE analysis (Fig. 2a) from affinity purification step showed a main band with estimated molecular weight of ~70 kDa and minor bands below it, present in the fraction corresponding to the major elution peak. Subsequent refolding was performed by stepwise dialysis to remove urea from protein sample. Taking into account, the protein impurities eluted after HisTrap column, a second chromatography with Sephacryl S-100 HR column was also performed. As a result, the final purity of the NS3 protein preparation obtained was 92 % of the total protein with a ∼44 % of final recovery (Table 1). The 70-kDa band detected by SDS-PAGE was identified as the recombinant DENV2-NS3 protein by WB using anti-His6x monoclonal antibody (Fig. 2b) and anti-NS3 protease polyclonal antibody (Fig. 2c).
Expression analysis of DENV2NS3 protein in Escherichia coli XL1-Blue cells: a 12 % SDS-PAGE, and b WB using anti-DENV2 HMAF. Lane 1 protein molecular weight marker (PM), prestained SDS-PAGE standards Bio-Rad, lane 2 E. coli culture containing recombinant plasmid after 4 h IPTG induction, lane 3 and 4, soluble and insoluble fractions of induced cell lysate, lane 5, protein sample in urea buffer before affinity chromatography
Purification and immunodetection of the recombinant NS3 protein: a 12 % SDS-PAGE, b WB using anti-His MAb, and c WB using anti-protease polyclonal antibody. Lane 1 Protein Marker, lane 2 protein sample in urea buffer protein before affinity chromatography, lane 3 pool of eluted fractions from HisTrap affinity column, lane 4 protein sample after refolding process, lane 5 pool of eluted fractions from Sephacryl S-100 HR column
Antigenic characterization
The antigenic properties of the purified recombinant protein were evaluated by an indirect ELISA using HMAFs against the four serotypes of DENV (Fig. 3). As a result, the recombinant NS3 protein exhibited a high recognition against the four HMAFs with an antibody titer ~1:100,000. On the other hand, the splenocytes obtained from DENV2 immunized mice were stimulated in vitro with the recombinant NS3 protein to evaluate its antigenic capacity by cytokine secretion. The highest levels of cytokines detected corresponded to IFN-γ secretion. Upon NS3 stimulation, IFN-γ levels (10,137 ± 4,781 pg/mL) were slightly higher than those observed to the DENV2 antigen positive control (4,806 ± 1,432 pg/mL). Also, IL-10 and TNF-α levels (71 and 32 respectively) detected upon stimulation with the recombinant protein were fold lower than IFN-γ levels. However, upon DENV2 stimulation, the secretion of TNF-α and IL-10 decreased 10- and 27-fold, respectively. The relation between IL-10 and TNF-α level was similar in both stimulations (Fig. 4).
Antibody response in mice
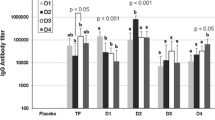
Fifteen days after the last dose, mice sera from both test and control groups were analyzed for the presence of anti-NS3 antibodies by ELISA. Table 2 shows the anti-NS3 antibody titer in NS3 group sera. Sera of immunized mice with NS3 display high ELISA reactivity. Anti-NS3 antibody titers between 1:16,000 and 1:64,000 showed sera from NS3 group, whereas antibody titers in sera from control group could not be detected. Isotype distribution determined by ELISA showed that IgG2b was the predominant subclass (Fig. 5).
Sera of mice immunized with the recombinant NS3 protein were able to react with native NS3 protein by WB showing the specificity of the response. Sera of negative control mice did not reacted with native NS3 protein. Characteristic bands corresponding to NS3, NS1, and E non-structural proteins were detected in the positive control strip with DENV2 HMAF.
The immunoblotting data were corroborated by IFA (Fig. 6b). Mice sera of NS3-immunized group, which yielded the highest anti-NS3 titers, were able to recognize the native NS3 protein in cells infected with DENV2. This result was indistinguishable from that observed in positive control cells using dengue 2- monoclonal antibody. Similar to non-inoculated cells, whose did not show immunofluorescence using dengue 2- monoclonal antibody, mice sera of negative control group failed to react with DENV2-inoculated cells.
Recognition of native NS3 protein from DENV2 by anti-recombinant NS3 antibodies elicited in mice using Western Blot (a) and indirect immunofluorescence assay, IFA, (b). a Strip blotting shows the native viral NS3 protein recognized by pool of NS3-immunized mice sera (line 2), but it was not recognized by pool of mice sera from negative control group (line 1). The control strip (line 3) shows the recognition of DENV proteins (NS3, NS1, and E) by anti-DENV2 HMAF. b Images in the upper panel, b 1 and b 2 , correspond to negative and positive cellular controls, respectively, for IFA using dengue 2- specific mouse monoclonal antibody 3H5-1-21 and anti-mouse IgG conjugated to FITC. Non-inoculated C6/36 cells (b 1 ) did not show immunofluorescence (in red), while positive immunofluorescence (in green) (b 2 ) was detected in DENV2-inoculated C6/36 cells. (b 3 ) Immunofluorescence was not observed in DENV2-inoculated C6/36 cells with a pool of mice sera from negative control group. However, green fluorescence was detected on DENV2-inoculated C6/36 cells using anti-recombinant NS3 antibodies elicited in mice (b 4 ). Photomicrographs were taken at ×400 (Color figure online)
Discussion
The need for a tetravalent dengue vaccine that confers protective immunity against the four serotypes and avoids the immune-enhancement mechanism has stimulated the use of some DENV proteins capable of inducing a cell-mediated immune response [14]. The multifunctional NS3 protein has been identified as the dominant target for CD8 + T cells. However, few studies have evaluated the immunogenicity of the NS3 protein for its use in a dengue vaccine formulation. In the present work, the antigenic and immunogenic properties of the recombinant DENV2-NS3 protein were evaluated. As a result, the NS3 protein was expressed in E. coli as inclusion bodies that were biochemically purified, and then refolded through a denaturation/renaturation procedure. Several authors have successfully expressed the NS3 protein in a soluble state [25, 36]. We obtained the recombinant NS3 protein associated with the insoluble fraction after cellular disruption, similar to results previously reported [37, 38]. Attempts to obtain the DENV2-NS3 protein soluble were carried out by our group using the pQE-30 vector. However, these were not successful even though some expression conditions such as IPTG concentration, temperature of culture, time of culture induction,and the presence of additives, were evaluated (submitted manuscript). Generally, overexpressed heterologous proteins are produced in E. coli as inclusion bodies. Most of dengue non-structural proteins expressed in bacterial cells have been found after cellular disruption in the insoluble fraction therefore leading to costly denaturation and refolding processes required to recover biologically active proteins [39, 40]. The strategy of two-step purification employed here allowed eliminating protein contaminants of low-molecular weight generated from either proteolytic degradation or internal initiation of the mRNA translation, co-purified with the desired NS3 protein. The refolding strategy was carried out by dialysis in the presence of CHAPS, which can suppress misfolding and the resultant aggregation reactions that occur during refolding. The reactivity of the purified protein with the four dengue HMAFs shown by ELISA, as well as from the native viral NS3 protein with sera from NS3-immunized mice by IFA demonstrated the specific recognizing and proper folding of the recombinant NS3 protein after the purification process. Additionally, the structural integrity of the purified DENV-2NS3 protein was also demonstrated by Western Blot. The presence in NS3 protein of epitopes conserved for all dengue viruses would be a desirable attribute for DENV diagnosis.
There are not many studies evaluating the use of the NS3 protein as an immunogenic antigen. In a previous report, the immunogenicity and antigenicity of a recombinant hepatitis C virus (HCV) NS3 protein was characterized. The authors found a high prevalence in immunized mice of anti-NS3 antibodies, which was explained by the large size of the NS3 protein together with the presence of multiple class II binding motifs. A similar explanation was suggested to the high frequency observed of NS3-specific antibodies in HCV-infected humans [41]. Taking into account that HCV and DENV belong to the Flavivirus family, we can suggest that the elevated reactivity and the high titers of anti-NS3 antibodies for DENV found in this work should be similarly explained. Recently, Alvarez-Rodriguez et al. [42] have expressed in E. coli the NS3 protease domain from all DENV serotypes. These proteins were immunogenic, inducing antibodies able of recognizing antigens up to a 1:3,200 dilution. In our study, the recombinant NS3 protein obtained induced even higher anti-NS3 antibody titers, a threefold more higher, than those shown by aforementioned authors [42]. Immunoglobulin isotype analysis of sera from mice immunized with the purified NS3 protein revealed the predominance of IgG2b, followed by IgG2a antibodies, which is signature of Th1 response. IgG2a and IgG2b are generally considered the most potent for activating effectors response and dominate antiviral immunity [43, 44]. Although the purified DENV2-NS3 protein was able to induce a strong IgG antibody response in mice and known literature report that anti-NS3 antibodies have been detected in sera of patients with primary and secondary DENV infections [42, 45], certainly these are not neutralizing antibodies.
The generation of neutralizing antibodies is a prerequisite for attaining adequate protection against DENV [46, 47]. Nevertheless, the cellular immune response is the principal arm of the adaptive immune system against non-cytopathic viruses such as dengue, as once the virus enters into the cell it is necessary to destroy it. Some characteristics of cellular response involved in protection versus severe disease have been proposed, which would be: the kinetics of the response; its magnitude; the balance of the Th response (Th1/Th2); and the specificity and avidity of the CTL response [48–50]. DENV-specific T cells recognized virus-infected cells and produced high levels of cytokines. These T cells produced IFNγ, TNFα, IL-2, and CC-chemokine ligand 4 (CCL4; also known as MIP1β) following a Th1 profile, whereas the production of Th2-type cytokines, such as IL-4, was less common [51]. Several authors have reported the CTL response role, specifically the contribution of CD8+ T cells, in limiting DENV infection. Yauch et al. [52] demonstrated using a novel mouse model that depletion of CD8+ T cells resulted in a lost of DENV infection clearance with significantly higher viral loads in the serum, spleen, and brain, suggesting that CD8 + T cells contribute to protection during primary DENV infection by reducing viral load. On the other hand, Williams et al. [53] showed the relevant role of the cellular immune response in protection against a secondary heterotypic DENV infection after treating mice with cyclophosphamide. The NS3 protein has been identified as the main target for T cell response, where epitopes present in its structure were recognized by murine and human CD8+CTLs [19, 54–56]. A recent study using overlapping peptides covering the whole DENV2 polyprotein found that the highest ex vivo T cell response were directed to the NS3 region [57]. Similarly, Rivino et al. [58] showed that during a DENV infection, the main targets of CD8 + T cells are the non-structural proteins (NS3 and NS5), while to CD4 + T cells the preferential targets are proteins recognized by B cells (E, C, and NS1). Furthermore, It has been demonstrated that DENV-specific and cross-reactive CTLs were induced upon mice immunization with NS3 peptides [59]. In particular, the induction of a protective response with the production of INF-γ by CD8 + T cells was detected in mice inoculated with NS3 DNA vaccines [60].
Our T cell data showed that there was a T cell response to recombinant antigen in vitro stimulation in splenocytes obtained from DENV2-immunized mice. A Th1 response was induced with an IFN-γ/TNF-α ratio in favor of IFN-γ. We also detected low levels of IL-10 (Th2 cytokine) produced by these T cells upon NS3 stimulation. The Th1 response is considered favorable to protection whereas a Th2 response has been suggested to be linked to more severe outcomes after natural infection [61]. It has been shown that pretreatment with IFNα and IFNγ or with IFNβ and IFNγ inhibits DENV replication in cells by preventing translation of viral RNA [62]. In contrast, TNFα may be directly associated with specific disease symptoms as suggested by the correlation between soluble TNF receptor concentrations and thrombocytopenia [63], as well as plasma leakage [64]. IL-10, a major anti-inflammatory cytokine, has been associated to a secondary infection and the severity of dengue infection [65–67]. Several studies have suggested the DHF may result from T cell-mediated immunopathology. The exacerbated production of inflammatory cytokines by memory T cells and the magnitude of T cell response have been associated with dengue disease severity [57, 68, 69]. However, Simmons et al. [70] in a study of Vietnamese adults with secondary DENV infection showed that neither the breadth nor magnitudes of the recorded peptide-specific T cell response, specifically NS3 peptides, were significantly associated with severity grade or the serotype of DENV mediating the current infection. On the other hand, the T cells involved in the pathogenesis show a cross-reactive recognition [71–73]. Consequently, our results emphasized the importance of NS3 as a T cell target, but these are not enough to conclude if the role of T cell response induced by the purified NS3 protein could determine the disease outcome during a natural infection.
In conclusion, we suggest that the recombinant NS3 protein expressed in E. coli acquired a correct conformation that was recognized by polyclonal antibodies against the four DENV serotypes. We also demonstrated that the purified NS3 protein conserve its antigenic determinants. Likewise, the recombinant NS3 protein induced high anti-recombinant antibody titers, preferentially IgG2b. In addition, this purified protein induced a cytokines profile dominated by IFN-γ over TNF-α upon NS3 stimulation of spleen cells from DENV2 immune mice. In general, these results suggest that a Th1 response could be elicited in mice by the recombinant NS3 protein. Hence, a vaccination strategy combining the induction of CD8 + T cell response to serotype-specific NS3 epitopes with a DENV structural protein target for neutralizing antibodies, for example the envelope Dom III fragment [74] should be considered an effective dengue vaccine to protect against all four serotypes. Further experiments are in progress by our group to evaluate the nature of cellular immune response and protective capacity of the recombinant DENV2-NS3 protein in mice upon DENV challenge.
Reference
S. Bhatt, P.W. Gething, O.J. Brady, J.P. Messina, A.W. Farlow, C.L. Moyes, J.M. Drake, J.S. Brownstein, A.G. Hoen, O. Sankoh, M.F. Myers, D.B. George, T. Jaenisch, G.R. Wint, C.P. Simmons, T.W. Scott, J.J. Farrar, S.I. Hay, Nature 496, 504–507 (2013)
T.J. Chambers, C.S. Hahn, R. Galler, C.M. Rice, Ann. Rev. Microbiol. 44, 649–688 (1990)
M.G. Guzman, M. Alvarez, S.B. Halstead, Arch. Virol. 158, 1445–1459 (2013)
R.F. Chen, K.D. Yang, L. Wang, J.W. Liu, C.C. Chiu, J.T. Cheng, Trans. R. Soc. Trop. Med. Hyg. 101, 1106–1113 (2007)
S.D. Jayaratne, V. Atukorale, L. Gomes, T. Chang, T. Wijesinghe, S. Fernando, G.S. Ogg, G.N. Malavige, BMC Res. Notes 5, 645 (2012)
S.B. Halstead, Vaccine 31, 4501–4507 (2013)
S.O. De Paula, D.M. Lima, R.F. de Oliveira Franca, A.C. Gomes-Ruiz, B.A. da Fonseca, Arch. Virol. 153, 2215–2223 (2008)
M.G. Guzman, R. Rodriguez, R. Rodriguez, L. Hermida, M. Alvarez, L. Lazo, M. Mune, D. Rosario, K. Valdes, S. Vazquez, R. Martinez, T. Serrano, J. Paez, R. Espinosa, T. Pumariega, G. Guillen, Am. J. Trop. Med. Hyg. 69, 129–134 (2003)
T.J. Kochel, K. Raviprakash, C.G. Hayes, D.M. Watts, K.L. Russell, A.S. Gozalo, I.A. Phillips, D.F. Ewing, G.S. Murphy, K.R. Porter, Vaccine 18, 3166–3173 (2000)
K. Raviprakash, D. Wang, D. Ewing, D.H. Holman, K. Block, J. Woraratanadharm, L. Chen, C. Hayes, J.Y. Dong, K. Porter, J. Virol. 82, 6927–6934 (2008)
L.E. Yauch, T.R. Prestwood, M.M. May, M.M. Morar, R.M. Zellweger, B. Peters, A. Sette, S. Shresta, J. Immunol. 185, 5405–5416 (2010)
L. Lazo, L. Hermida, A. Zulueta, J. Sanchez, C. Lopez, R. Silva, G. Guillen, M.G. Guzman, Vaccine 25, 1064–1070 (2007)
L. Gil, L. Bernardo, A. Pavon, A. Izquierdo, I. Valdes, L. Lazo, E. Marcos, Y. Romero, M.G. Guzman, G. Guillen, L. Hermida, J. Gen. Virol. 93, 1204–1214 (2012)
L. Gil, C. Lopez, A. Blanco, L. Lazo, J. Martin, I. Valdes, Y. Romero, Y. Figueroa, G. Guillen, L. Hermida, Viral Immunol. 22, 23–30 (2009)
L. Gil, C. Lopez, L. Lazo, I. Valdes, E. Marcos, R. Alonso, A. Gambe, J. Martin, Y. Romero, M.G. Guzman, G. Guillen, L. Hermida, Int. Immunol. 21, 1175–1183 (2009)
B. Guy, N. Nougarede, S. Begue, V. Sanchez, N. Souag, M. Carre, L. Chambonneau, D.N. Morrisson, D. Shaw, M. Qiao, R. Dumas, J. Lang, R. Forrat, Vaccine 26, 5712–5721 (2008)
I. Kurane, L. Zeng, M.A. Brinton, F.A. Ennis, Virology 240, 169–174 (1998)
K.M. Mladinich, S.M. Piaskowski, R. Rudersdorf, C.M. Eernisse, K.L. Weisgrau, M.A. Martins, J.R. Furlott, C.D. Partidos, J.N. Brewoo, J.E. Osorio, N.A. Wilson, E.G. Rakasz, D.I. Watkins, Immunogenetics 64, 111–121 (2012)
A.C. Spaulding, I. Kurane, F.A. Ennis, A.L. Rothman, J. Virol. 73, 398–403 (1999)
A. Mathew, I. Kurane, S. Green, H.A. Stephens, D.W. Vaughn, S. Kalayanarooj, S. Suntayakorn, D. Chandanayingyong, F.A. Ennis, A.L. Rothman, J. Virol. 72, 3999–4004 (1998)
I. Kurane, T. Matsutani, R. Suzuki, T. Takasaki, S. Kalayanarooj, S. Green, A.L. Rothman, F.A. Ennis, Trop. Med. Health 39, 45–51 (2011)
V. Berrios, I. Kurane, F.A. Ennis, Immunol. Invest. 25, 231–240 (1996)
A.L. Rothman, N. Kanesa-thasan, K. West, J. Janus, J.F. Saluzzo, F.A. Ennis, Vaccine 19, 4694–4699 (2001)
D. Luo, T. Xu, C. Hunke, G. Gruber, S.G. Vasudevan, J. Lescar, J. Virol. 82, 173–183 (2008)
J. Li, S.P. Lim, D. Beer, V. Patel, D. Wen, C. Tumanut, D.C. Tully, J.A. Williams, J. Jiricek, J.P. Priestle, J.L. Harris, S.G. Vasudevan, J. Biol. Chem. 280, 28766–28774 (2005)
D. Luo, T. Xu, R.P. Watson, D. Scherer-Becker, A. Sampath, W. Jahnke, S.S. Yeong, C.H. Wang, S.P. Lim, A. Strongin, S.G. Vasudevan, J. Lescar, EMBO J. 27, 3209–3219 (2008)
T. Xu, A. Sampath, A. Chao, D. Wen, M. Nanao, P. Chene, S.G. Vasudevan, J. Lescar, J. Virol. 79, 10278–10288 (2005)
G. Kuno, D.J. Gubler, M. Velez, A. Oliver, Bull. World Health Organ. 63, 279–286 (1985)
W.E. Brandt, E.L. Buescher, I.M. Hetrick, Am. J. Trop. Med. Hyg. 16, 339–347 (1967)
G. Kouri, P. Mas, M.G. Guzman, M. Soler, A. Goyenechea, L. Morier, Bull. Pan Am. Health Organ. 17, 126–132 (1983)
V. Churdboonchart, N. Bhamarapravati, S. Peampramprecha, S. Sirinavin, Am. J. Trop. Med. Hyg. 44, 481–493 (1991)
U.K. Laemmli, Nature 227, 680–685 (1970)
M.M. Bradford, Anal. Biochem. 72, 248–254 (1976)
H. Towbin, T. Staehelin, J. Gordon, Proc. Natl. Acad. Sci. USA 76, 4350–4354 (1979)
E.A. Henchal, M.K. Gentry, J.M. McCown, W.E. Brandt, Am. J. Trop. Med. Hyg. 31, 830–836 (1982)
G. Bartelma, R. Padmanabhan, Virology 299, 122–132 (2002)
T.L. Arakaki, N.X. Fang, D.P. Fairlie, P.R. Young, J.L. Martin, Protein Expr. Purif. 25, 241–247 (2002)
Z. Chen, Y. Tian, L. Liu, J. An, Hybridoma (Larchmt) 27, 467–471 (2008)
J.H. Amorim, B.F. Porchia, A. Balan, R.C. Cavalcante, S.M. da Costa, A.M. de Barcelos Alves, L.C. de Souza Ferreira, J. Virol. Methods 167, 186–192 (2010)
Q. Huang, A.S. Chen, Q. Li, C. Kang, Protein Expr. Purif. 80, 169–175 (2011)
M. Sallberg, Z. Zhang, M. Chen, L. Jin, A. Birkett, D.L. Peterson, D.R. Milich, J. Gen. Virol. 77, 2721–2728 (1996)
L.M. Alvarez-Rodriguez, A. Ramos-Ligonio, J.L. Rosales-Encina, M.T. Martinez-Cazares, A. Parissi-Crivelli, A. Lopez-Monteon, J. Trop. Med. 2012, 956875 (2012)
D. Markine-Goriaynoff, J.P. Coutelier, J. Virol. 76, 432–435 (2002)
F. Nimmerjahn, J.V. Ravetch, Science 310, 1510–1512 (2005)
K. Valdes, M. Alvarez, M. Pupo, S. Vazquez, R. Rodriguez, M.G. Guzman, Clin. Diagn. Lab. Immunol. 7, 856–857 (2000)
B.R. Murphy, S.S. Whitehead, Annu. Rev. Immunol. 29, 587–619 (2011)
J.L. Kyle, S.J. Balsitis, L. Zhang, P.R. Beatty, E. Harris, Virology 380, 296–303 (2008)
M.M. Mangada, T.P. Endy, A. Nisalak, S. Chunsuttiwat, D.W. Vaughn, D.H. Libraty, S. Green, F.A. Ennis, A.L. Rothman, J. Infect. Dis. 185, 1697–1703 (2002)
S. Green, A. Rothman, Curr. Opin. Infect. Dis. 19, 429–436 (2006)
M.M. Mangada, A.L. Rothman, J. Immunol. 175, 2676–2683 (2005)
A.L. Rothman, Nat. Rev. Immunol. 11, 532–543 (2011)
L.E. Yauch, R.M. Zellweger, M.F. Kotturi, A. Qutubuddin, J. Sidney, B. Peters, T.R. Prestwood, A. Sette, S. Shresta, J. Immunol. 182, 4865–4873 (2009)
K.L. Williams, S. Zompi, P.R. Beatty, E. Harris, Ann. N. Y. Acad. Sci. 1171(Suppl 1), E12–E23 (2009)
P.G. Livingston, I. Kurane, L.C. Dai, Y. Okamoto, C.J. Lai, R. Men, S. Karaki, M. Takiguchi, F.A. Ennis, J. Immunol. 154, 1287–1295 (1995)
A. Mathew, I. Kurane, A.L. Rothman, L.L. Zeng, M.A. Brinton, F.A. Ennis, J. Clin. Invest. 98, 1684–1691 (1996)
M. Lobigs, C.E. Arthur, A. Mullbacher, R.V. Blanden, Virology 202, 195–201 (1994)
T. Duangchinda, W. Dejnirattisai, S. Vasanawathana, W. Limpitikul, N. Tangthawornchaikul, P. Malasit, J. Mongkolsapaya, G. Screaton, Proc. Natl. Acad. Sci. USA 107, 16922–16927 (2010)
L. Rivino, E.A. Kumaran, V. Jovanovic, K. Nadua, E.W. Teo, S.W. Pang, G.H. Teo, V.C. Gan, D.C. Lye, Y.S. Leo, B.J. Hanson, K.G. Smith, A. Bertoletti, D.M. Kemeny, P.A. MacAry, J. Virol. 87, 2693–2706 (2013)
H. Masaki, Y. Fujii, C. Wakasa-Morimoto, T. Toyosaki-Maeda, K. Irimajiri, T.T. Tomura, I. Kurane, Virus Res. 144, 188–194 (2009)
S.M. Costa, A.P. Yorio, A.J. Goncalves, M.M. Vidale, E.C. Costa, R. Mohana-Borges, M.A. Motta, M.S. Freire, A.M. Alves, PLoS ONE 6, e25685 (2011)
U.C. Chaturvedi, R. Agarwal, E.A. Elbishbishi, A.S. Mustafa, FEMS Immunol. Med. Microbiol. 28, 183–188 (2000)
M.S. Diamond, E. Harris, Virology 289, 297–311 (2001)
D.H. Libraty, T.P. Endy, H.S. Houng, S. Green, S. Kalayanarooj, S. Suntayakorn, W. Chansiriwongs, D.W. Vaughn, A. Nisalak, F.A. Ennis, A.L. Rothman, J. Infect. Dis. 185, 1213–1221 (2002)
S. Green, D.W. Vaughn, S. Kalayanarooj, S. Nimmannitya, S. Suntayakorn, A. Nisalak, R. Lew, B.L. Innis, I. Kurane, A.L. Rothman, F.A. Ennis, J. Infect. Dis. 179, 755–762 (1999)
A.B. Perez, G. Garcia, B. Sierra, M. Alvarez, S. Vazquez, M.V. Cabrera, R. Rodriguez, D. Rosario, E. Martinez, T. Denny, M.G. Guzman, J. Med. Virol. 73, 230–234 (2004)
A.B. Perez, B. Sierra, G. Garcia, E. Aguirre, N. Babel, M. Alvarez, L. Sanchez, L. Valdes, H.D. Volk, M.G. Guzman, Hum. Immunol. 71, 1135–1140 (2010)
K.I. Schexneider, E.A. Reedy, Curr. Hematol. Rep. 4, 145–148 (2005)
J. Mongkolsapaya, W. Dejnirattisai, X.N. Xu, S. Vasanawathana, N. Tangthawornchaikul, A. Chairunsri, S. Sawasdivorn, T. Duangchinda, T. Dong, S. Rowland-Jones, P.T. Yenchitsomanus, A. McMichael, P. Malasit, G. Screaton, Nat. Med. 9, 921–927 (2003)
I. Zivna, S. Green, D.W. Vaughn, S. Kalayanarooj, H.A. Stephens, D. Chandanayingyong, A. Nisalak, F.A. Ennis, A.L. Rothman, J. Immunol. 168, 5959–5965 (2002)
C.P. Simmons, T. Dong, N.V. Chau, N.T. Dung, T.N. Chau, T.T. le Thao, N.T. Dung, T.T. Hien, S. Rowland-Jones, J. Farrar, J. Virol. 79, 5665–5675 (2005)
R. Appanna, T.L. Huat, L.L. See, P.L. Tan, J. Vadivelu, S. Devi, Clin. Vaccine Immunol. 14, 969–977 (2007)
C.M. Beaumier, A. Mathew, H.S. Bashyam, A.L. Rothman, J. Infect. Dis. 197, 608–617 (2008)
H. Friberg, L. Burns, M. Woda, S. Kalayanarooj, T.P. Endy, H.A. Stephens, S. Green, A.L. Rothman, A. Mathew, Immunol. Cell Biol. 89, 122–129 (2011)
L. Hermida, L. Bernardo, J. Martin, M. Alvarez, I. Prado, C. Lopez, L. Sierra Bde, R. Martinez, R. Rodriguez, A. Zulueta, A.B. Perez, L. Lazo, D. Rosario, G. Guillen, M.G. Guzman, Vaccine 24, 3165–3171 (2006)
Acknowledgments
We would like to thank the International Society for Infectious Diseases (ISID) for providing financial support supports through an ISID Exchange Fellowship to Rosa Ramirez. We are grateful to technician Ramón Linares for his valuable help in the care and maintenance of the animals during the experiment. We also thank Melkis Alfonso, Osmel Fleitas and Tatiana Almaguer for the technical assistance. This study was also jointly supported by funds from the Cuban government, and CAPES, agency from de Brazilian government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramírez, R., Falcón, R., Izquierdo, A. et al. Recombinant dengue 2 virus NS3 protein conserves structural antigenic and immunological properties relevant for dengue vaccine design. Virus Genes 49, 185–195 (2014). https://doi.org/10.1007/s11262-014-1087-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-014-1087-3