Abstract
Recent outbreaks of orf in China have been attributed to a novel strain of Orf virus (ORFV) designated ORFV-Jilin. Currently, monoclonal antibodies (Mabs) have not yet been developed against this specific pathogen even though such entities could have potential applications regarding the diagnosis and characterization of ORFV-Jilin. Therefore, the current study was undertaken to generate Mab against the immunodominant ORFV059 protein of this virus. For this purpose, the ORFV-Jilin ORFV059 protein was expressed in Escherichia coli and subsequently used as an antigen to immunize mice and for the initial screening of hybridomas prepared from the mice for their ability to produce anti-ORFV059 protein Mabs via an indirect ELISA. Ten, positive hybridomas were identified in this manner and verified based on the ability of their released Mab to react specifically with both naturally and artificially expressed ORFV059 protein in Western blots. The two hybridomas with the greatest propensity to secrete Mab were subcloned three times before being introduced intraperitoneally into mice. Afterwards, both Mab were separately purified from the mice’s ascetic fluids and found to successfully recognize the ORFV-Jilin ORFV059 protein in a variety of immunological assays. Thus, the widespread utility of these Mab as a diagnostic core reagent should prove invaluable for further investigations regarding the mechanisms of orf pathogenesis and the control of this disease. In this regard, it should be noted that Mab A3 was used to confirm the predicted late expression of the ORFV-Jilin ORFV059 protein during virus replication.
Similar content being viewed by others
Introduction
Orf, also known as contagious ecthyma or scabby mouth, is a zoonotic infectious disease that mainly affects sheep, goats, wild ruminants and humans with a worldwide distribution [1–3]. The disease is usually manifested as maculopapular, vesicular, pustular and proliferative lesions on the lips, muzzle, nostrils, teats, and oral mucosa of sheep, goats and wild animals [2] and as self-limiting, painful pustular lesions on the hands and fingers of humans [3]. The lesions can develop into erythema, vesicles, pustules and scabs around the virus entry sites [4]. Partial protection against the primary infection is provided by a T-helper type I response and the lesions usually resolved within 6–8 weeks after their initial appearance. Secondary infections can occur and the resultant lesions are smaller in size and resolve sooner than those arising during a primary infection [5–7].
The causative agent of orf is the orf virus (ORFV) and this epitheliotropic virus is the type species of the Parapoxvirus genus [8]. Parapoxviruses have a particular ovoid shape with a crisscross pattern on the particle’s surface [9]. The genome of ORFV is 138 kb with G + C content up to 64% and contains 132 putative genes [10]. Analysis of several strains of ORFV has revealed that the ORFV059 gene encodes an immunodominant protein that is expressed on the intracellular, mature virion envelop and shares structure similarities with the vaccinia H3L immunodominant protein and other poxvirus homologs [9–11]. The ORFV059 protein is a member of the C-terminal anchor family and is involved in virus adsorption, maturation [12, 13], and pathogenesis [11, 14, 15]. Moreover, this protein has been used in the development of diagnostic reagents [15] and a subunit vaccine [16]. Consequently, the ORFV059 protein is favored as an antigen for the production of antibodies.
Recently, outbreaks of orf in sheep have occurred in Jilin and other provinces of Northern China [17, 18]. So far, characterization of the responsible strain of ORFV (ORFV-Jilin) has been limited and the commercial availability of only one monoclonal antibody (Mab) raised against the ORFV059 protein of a different ORFV strain has hindered investigations regarding the prevalence of ORFV-Jilin in China. To correct this deficiency, we have set about in the present study to generate Mab capable of recognizing ORFV-Jilin. Initially, we amplified the ORFV059 gene from the ORFV-Jilin genome [17] and inserted it into a bacterial expression vector. The expressed, recombinant protein (rORFV059) was used as an antigen in the production/identification of two mouse hybridomas that secreted anti-ORFV059 protein Mab. Subsequent analysis of both purified Mab indicated that either one could be used for the detection of the ORFV-Jilin ORFV059 protein by using ELISA, Western blotting, immunoprecipitation, immunofluorescence, immunocytochemistry, or immunohistochemistry. Thus, these two Mab should find widespread application in the diagnosis of orf in China and perhaps other countries and in molecular biological studies involving at least the ORFV-Jilin strain.
Materials and methods
Cells and virus
Primary ovine fetal turbinate (OFTu) cells served as a host for ORFV and were cultured in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), containing l-glutamine (2 mM), gentamicin (50 μg/ml), penicillin (100 U/ml), and streptomycin (100 μg/ml). The ORFV strain used in this study (ORFV-Jilin) was isolated from a sheep afflicted with orf in the Jilin province of China [17]. For the cross reactivity study, different ORFV strains were used (OV-IA82 [10], ORFV-GO-BT [19], ORFV-Nongan and ORFV-Taian recently isolated and identified from different area of China, Table 2). Vaccinia virus Tiantan strain [20], Goatpoxvirus [19] and Fowlpoxvirus F2 and F3 strains [21] were also used for the cross reactivity study.
Purification of ORFV-Jilin
Upon reaching 90% confluency, monolayers of OFTu cells grown in 10 T150 tissue culture flasks were infected with the ORFV-Jilin isolate at a MOI of 1. After 3–5 days, when CPE was evident as most of the cells exhibited a rounded morphology, the overlying medium was clarified during centrifugation at 1,500 rpm and 4°C for 10 min. The supernatant containing the mature viral particles was then subjected to sucrose gradient ultracentrifugation [22, 23]. The band containing the viral particles was removed and the virions were heat-inactivated during incubation at 96°C for 90 min, sonicated, and stored at −80°C. The protein concentration of the purified virus was measured with the Bio-Rad protein assay reagent (Bio-Rad Laboratories, Inc.).
Generation of expression plasmids
For bacterial expression of the ORFV059 protein, the intact ORFV059 gene was amplified using PCR from the ORFV-Jilin genome in the presence of primers ORFV059Fw1: 5′-CATTAACCATGGATCCACCCGAAATCACGGC-3′ and ORFV059Rv1: 5′-AATCATCTCGAGCACGATGGCCGTGACCAGCAGC-3′ that contained unique Nco I or Xho I restriction sites (underlined), respectively, near their 5′ termini. The resultant amplicon was inserted into the pET28a (+) vector (Novagen) via Nco I/Xho I restriction sites to generate pET28-ORFV059. For expression of the ORFV059 protein in mammalian cells, its complete coding sequence was similarly amplified from ORFV-Jilin DNA utilizing the same reverse primer described above and a novel forward primer, ORFV059Fw2: 5′-CATATCGAATTCATGGATCCACCCGAAATCACGGC-3′ having a unique EcoRI restriction site near its 5′ terminus. In this case, the restriction enzyme-digested amplicon was cloned in-frame with a Flag-tag in the vector pCMVtag4A (Clontech) to generate pCMV-ORFV059-Flag. Afterwards, the nucleotide sequence integrity of both constructs was verified by sequencing before use.
Purification of ORFV-Jilin ORFV059 protein expressed in bacteria
Recombinant ORFV-Jilin ORFV059 (rORFV059) protein was prepared, purified and quantified essentially as previously described [24, 25]. In brief, the His-tagged, rORFV059 protein was expressed in Escherichia coli BL21 transformed with pET28-ORFV059 during induction in the presence of 0.5 mM IPTG for 12 h at 30°C. After the bacteria were concentrated by centrifugation at 5,000×g and 4°C for 15 min, the pellet was resuspended in 5 ml/g lysis buffer (PBS, pH 7.4, containing 0.1% IGEPAL and 2.5% glycerol) and sonicated. Inclusion bodies (IB) were pelleted from the suspension during a 15 min centrifugation at 6,000×g and 4°C and then resuspended in lysis buffer containing 0.4 mg/ml lysozyme to the same volume as used in the initial resuspension. After a 15-min incubation at ambient temperature, 20 ml of 1:20 dilution of lysis buffer was added to the suspension and was then centrifuged for 15 min at 6,000×g and 4°C. The pellet was then resuspended in 20 ml solubilization buffer (50 mM Tris, pH 8.0, 6 M urea, 0.4 M NaCl, 10 mM 2-mercaptoethanol) and sonicated on ice. The IB suspension was solubilized during shaking for 1 h at ambient temperature and centrifuged at 14,000×g and 4°C for 1 h. The supernatant that contained the solubilized but denatured rORFV059 protein was diluted with 20 ml equilibration buffer (50 mM Tris, pH 8.0, 6 M urea, 0.4 M NaCl, 10 mM 2-mercaptoethanol, 5 mM imidazole) and applied to equilibrated nickel column. The column was then washed consecutively with 20 ml of the following buffers: once with 50 mM Tris, pH 8.0, 6 M urea, 0.5 M NaCl, 10 mM 2-mercaptoethanol, 10 mM imidazole; once with 50 mM Tris, pH 8.0, 6 M urea, 10 mM 2-mercaptoethanol, 10 mM imidazole; and then twice with 50 mM Tris, pH 8.0, 6 M urea, 10 mM 2-mercaptoethanol, 20 mM imidazole. Proteins still attached to the resin were eluted in five 5 ml washes with 50 mM Tris, pH 8.0, 6 M urea, 10 mM 2-mercaptoethanol, 100 mM EDTA. Each fraction was analyzed for the presence of rORFV059 protein by using SDS-PAGE followed by staining of the gel with GelCode Blue. The rORFV059 protein-containing fractions were combined, desalted in a PD-10 column, eluted with 50 mM Tris, pH 8.0, containing 10% glycerol, and again tested for the presence of rORFV059 protein using the above-described method. The protein concentration of the final preparation was determined using the Bio-Rad protein assay reagent.
Immunization of mice with rORFV059 protein and establishment of hybridomas
Ten 8-week-old female BALB/c mice were immunized intraperitoneally (i.p.) with 50 μg of rORFV059 protein plus bentonite. 4 weeks after priming, mice were boosted four times in 2 week intervals by i.p. injection of 50 μg rORFV059 protein per mouse. Spleen cells were isolated from the immunized mice, washed twice with PBS, and then mixed and fused with logarithmically growing SP2/0 myeloma cells in a ratio of 5:1 in the presence of polyethylene glycol 3500 (Roche). The treated cells were resuspended in RPMI 1640 medium supplemented with 20% FBS, 10 U/ml IL-6 (Roche), OPI media supplement (Sigma-Aldrich), and HAT media supplement (Sigma-Aldrich) and plated into 96-well tissue culture plates at a density of 1.2 × 105 cells per well in a volume of 200 μl. After cultivation at 37° C with 5.0% CO2 for 7 to 10 days, the culture medium in each well was analyzed using an indirect ELISA for the presence of anti-ORFV059 Mab. Two of the hybridomas, A3 and G3, were subjected to limiting dilution three times before being separately amplified in mice. In this case, each member of a group of five pristine-treated mice received 8 × 105 cells in 0.5 ml PBS by i.p. injection. After 10 days, ascitic fluid was collected from the mice that had been placed under terminal halothane anesthesia, centrifuged at 12,000 rpm and 4°C for 10 min to remove cells and debris and then stored at −20°C. Later, both Mabs were affinity purified using Protein G Sepharose 4 Fast Flow as recommended by the manufacturer (GE Healthcare).
Enzyme-linked immunosorbent assay (ELISA)
Wells in ELISA plates were each coated with 100 μl of purified rORFV059 protein (1 μg/ml) or ORFV-Jilin (500 ng/ml) at 4°C overnight, blocked for 2 h at 4°C with 1% BSA, and incubated for 1 h at 37°C with 100 μl of hybridoma cultured medium. After washing with PBS, the wells were incubated for 30 min with goat anti-mouse antibodies labeled with horseradish peroxidase (HRP), washed four times with PBS, incubated with ABTS substrate (1112597, Roche, Mannheim, Germany) for 10 min and the OD405 of the contained solutions measured.
Immunoglobulin (Ig) isotyping of anti-ORFV059 protein Mab
The Ig isotyping of the obtained mouse Mabs was performed using the Roche Antibody Isotyping Kit (Roche).
Transfections
Sub-confluent monolayers of OFTu cells were transfected with 2 μg of either pCMVTag4A or pCMV-ORFV059-Flag in the presence of Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After 24 h, the cells were washed with PBS and incubated in 1 ml cell lysis buffer (25 mM Tris–HCl, pH 7.4, 250 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and protease and phosphatase inhibitors) for 20 min on ice. The protein concentrations of the lysates were determined using the Bio-Rad protein assay reagent.
Western blots and immunoprecipitations
For Western blotting, 2 μg of purified ORFV-Jilin protein or 1 μg of purified rORFV059 protein were individually electrophoresed in 10% SDS-PAGE gels and then transferred to nitrocellulose membranes. Blots were probed with the cultured medium from the each of the ten hybridomas deemed to produce anti-ORFV059 protein Mab or with serum collected from ORFV-infected sheep in the Jiling province of Northern China, washed, incubated with goat anti-mouse HRP-conjugated IgG antibody (Santa Cruz), washed and then developed using a chemiluminescent substrate (ECL, Pierce-Thermo Scientific). Alternately, blots prepared using individual, 20 μg samples of lysate prepared from OFTu cells transfected 24 h earlier with either pCMVTag4A or pCMV-ORFV059-Flag were probed with either purified Mab A3 or G3 or anti-GADPH antibody.
For immunoprecipitation, OFTu cells were first transiently transfected with parental plasmid, pCMVTag4A, or the recombinant plasmid, pCMV-ORFV059-Flag. At 24 h post-transfection, the cells were harvested in 1 ml of PBS and incubated in cell lysis buffer for 20 min on ice. Protein extracts were allowed to react with purified Mab A3 or G3 for 3 h at 4°C and subsequently incubated overnight at 4°C with 50 μl of protein G agarose beads (Upstate). Samples were washed three times with lysis buffer and the immunoprecipitated proteins were resolved in 10% SDS-PAGE gels and transferred to nitrocellulose membranes. Blots were probed with anti-Flag M2 Mab (Sigma) and developed as described above.
Immunofluorescence
OFTu cells cultured on glass coverslips were infected with ORFV-Jilin at a MOI of 5, fixed with 4% formaldehyde solution at 0, 4, 6, 8, 10, 12 and 24 h postinfection (p.i.), and permeabilized with 0.25% Triton X-100 for 10 min at ambient temperature. After being blocked with PBS containing 1% bovine serum albumin, cells were incubated with Mab A3 or G3 for 1 h at ambient temperature. Unbound antibodies were washed and samples incubated with anti-mouse IgG Alexa Fluor 488 (Green) for 1 h at room temperature, stained with 4′,6′-diamidino-2-phenylindole (DAPI) for 10 min and examined using a Leica inverted fluorescence microscope.
Reverse transcription-PCR (RT-PCR) of ORFV gene transcripts
OFTu cells were cultured in 35-mm dishes for 16 h and then infected with ORFV-Jilin at a MOI of 10 in the presence or absence of cytosine arabinoside (AraC; 40 μg/ml; Sigma Aldrich, St. Louis, MO), an inhibitor of DNA replication and, consequently, of late poxvirus transcription [26]. Cells were harvested at 0, 1, 2, 3, 6, 12, and 24 h p.i., and total RNA was extracted using TRIzol reagent (Invitrogen). Samples were treated with DNase I (New England Biolabs) for 10 min at 37°C, and the RNA was purified using a RNeasy minikit (Qiagen). 2 μg RNA per sample was reverse transcribed by using a Superscript III kit (Invitrogen), following the manufacturer’s protocol in the presence of the primer oligo-dT-5′-ATGGATGCCTACAGCGCTCTTTTTTTTTTTTTTTTTTTT-3′. Amplicons corresponding to a portion of the converted ORFV059 or ORFV024 gene [27] transcripts were obtained by standard PCR utilizing 1 μl of the completed RT reaction and the following primer pairs: 024intFw-5′-GCGGACACAGCCACAACCACAGTC-3′ and 024intRv-5′-CTAGCACGCGCTTTCGGTACCGCC-3′ or 059Fw (5′-ATGGATCCACCCGAAATCAC-3′), and 059Rv (5′-TCACACGATGGCCGTGACCAGC-3′), respectively. The results are representative of three independent experiments.
Immunocytochemistry
For immunocytochemistry, the purified, ORFV-Jilin particles were fixed for 60 min at 4°C in a solution containing 0.1% glutaraldehyde, 4% freshly prepared formaldehyde, 1% picric acid and 3.5% sucrose in 0.1 M cacodylate buffer pH 7.2. The fixed viruses were collected on 300 mesh nickel grids, permeabilized with 0.25% Triton X-100 for 10 min at ambient temperature, and blocked for 30 min with PBS containing 0.1% Tween 20 and 0.5% cold fish gelatin (PBS-TW-FG). Grids were incubated with Mab A3 or G3 at ambient temperature for 2 h, washed with PBS-TW-FG, incubated for 1 h with PBS-TW-FG containing a goat anti-mouse IgG (1:50) labeled with 10 nm gold particles (Sigma), incubated for 15–20 min in PBS-2.5 M NaCl, washed in distilled water, stained with uranyl acetate and lead citrate, and examined with a transmission electron microscope.
Virus neutralization assays
The ability of Mab A3 and G3 to neutralize the infectivity of ORFV-Jilin was examined using a modified fluorescent focus neutralization assay [28]. Briefly, 50 μl of four ten-fold dilutions of purified Mab A3 and G3, normal mouse serum in MEM supplemented with 5% bovine fetal serum or just MEM supplemented with 5% bovine fetal serum were added to individual wells of a 96-well plate. An equal volume of virus, at a concentration of 2000 TCID50/ml, was added to each well and after 1 h at 37°C, the total mix (100 μl) was transferred to separate wells of a 96-well plate containing confluent monolayers of OFTu cells. After 24 h, the monolayers were fixed with 4% formaldehyde at ambient temperature for 1 h and the infected cells were detected based on reactivity with Mab A3 or G3 as discerned using the Abcam immunofluorescence protocol (www.abcam.com/technical). The fluorescent foci were observed and counted using an inverted fluorescent microscope. The percentage of ORFV neutralization was defined according to the following formula: [(PNV − PNS)/PNV] × 100, where PNV was the average number of plaques made by the untreated ORFV and PNS was the average number of plaques made by the ORFV after exposure to Mab A3, Mab G3 or normal mouse serum. Statistics were performed on the results of three independent experiments using a two-tailed, unpaired t test with 95% confidence bounds.
Immunohistochemistry (IHC)
Samples of skin that exhibited morphological changes characteristic of ORFV infection were removed from a diseased sheep and cut into 4 μm thick sections. These sections were mounted on slides pre-treated with silicon, exposed to 3% hydrogen peroxide for 15 min at ambient temperature to quench any endogenous peroxidase activity and washed four times with PBS, pH 7.2, at 5 min intervals. The sections were then blocked with PBS containing 5% BSA for 15 min at ambient temperature, incubated at 4°C overnight with either purified Mab A3 or G3 diluted 1,000-fold in PBS containing 1% BSA or with diluted normal mouse serum, washed with three times with PBS at 5 min intervals and incubated for 4 h at ambient temperature with a HRP-conjugated rabbit anti-mouse IgG antibody (Cell Signal) diluted 1:2,000 in PBS containing 1% BSA. After again washing the sections three times with PBS at 5 min intervals, positive reactions in the tissues were visualized after incubation of the sections in a working solution of the DAB (3,3′-diaminobenzidine-tetrahydrochloride) Substrate Kit for Peroxidase (Promega, WI) for 15 min at ambient temperature. Afterwards, the slides were counterstained with hematoxylin, washed in PBS for 10 min, and mounted with GVA medium (Promega).
Results
Characterization and purification of the ORFV-Jilin ORFV059 protein
Due to the demonstrated variability in the predicted amino acid sequences of the protein encoded by the ORFV059 gene of various strains of ORFV (17), it was decided to utilize the authentic ORFV-Jilin homolog as an antigen for hybridoma production. Thus, the gene coding for the ORFV059 protein was PCR amplified from the ORFV-Jilin genome in the presence of primers that corresponded to sites flanking the region of interest. The resultant amplicon contained an open reading frame of 1,014 nucleotides that corresponded to the ORFV-Jilin ORFV059 gene and was inserted into a bacterial expression vector to create pET28-ORFV059. Interestingly, the encoded protein lacked amino acids P and A present at positions 41 and 42, respectively, of its counterpart in another ORFV strain, OV-IA82, and also had the following amino acid substitutions, S46K, A50S, P127S, F135I, I136V, F145L, K159R, A160T, T165A, and S205N (Fig. 1). Subsequently, a His-tagged form of the ORFV-Jilin ORFV059 (rORFV059) protein having a molecular weight of 39 kDa was highly expressed in E. coli transformed with pET28-ORFV059 and purified using an affinity nickel column under denaturing conditions (Fig. 2).
Comparison of the predicted amino acid sequences of the ORFV059 protein of ORFV-Jilin and ORFV OV-IA82. The predicted amino acid sequences of the ORFV059 protein expressed by the ORFV-Jilin and ORFV OV-IA82 strains were based on the nucleotide sequences of the corresponding gene as determined here (GenBank accession no. FJ808075) and previously (GenBank accession no. AY386263.1) [10, 18]. These sequences were aligned using the CLUSTAL W program (San Diego Supercomputer Center biology workbench: http://workbench.sdsc.edu/)
Expression and purification of rORFV059. IB prepared from E. coli transformed with pET28-ORFV059 were solubilized (IBS), applied to a Ni2+–NTA column and the flow-through (F) retained. Afterwards, the column was washed sequentially with Tris-based buffers containing urea, 2-mercaptoethanol and: 0.5 M NaCl, 10 mM imidazole (W1); 10 mM imidazole (W2); 20 mM imidazole (W3 and W4); or 100 mM EDTA (E1-E5). Aliquots of each fraction were electrophiresed in 12.5% SDS-PAGE and the separated proteins were stained with GelCode Blue. The positions of the protein molecular weight markers are indicated on the left-hand side of the figure while the location of an approximately 39 kDa protein corresponding in molecular weight to rORFV059 is shown on the right-hand side of the figure
Preparation and characterization of anti-ORFV059 protein Mab
Approximately 380 hybridomas prepared from the splenocytes of mice previously exposed to the rORFV059 protein were screened for the ability to secrete Mab that recognized both rORFV059 protein and gradient-purified ORFV-Jilin in separate wells of an indirect ELISA. Subsequently, the specificity of the antibodies in the ten hybridoma culture supernatants that exhibited the greatest reactivity in the two ELISAs (Table 1) was verified using these media for Western blotting of the separated ORFV proteins. Despite the observance of multiple bands of varying molecular weight in this blot when the serum from either of two ORFV-positive sheep was used as an antibody source (Fig. 3a, lanes S1, S2), only a prominent band of approximately 39 kDa, corresponding in size to that of the recognized rORFV059 protein (Fig. 3b) was detected (Fig. 3a, lanes A3–H5). Although any of these ten hybridomas should have proven satisfactory for further Mab development, only those two (A3 and G3) whose culture medium was associated with the highest ELISA absobance450 values (Table 1) were subcloned and used to produce Mab in the ascites fluid of mice. As described above, recognition by Mab released by the cloned hybridomas as well as the Mab A3 and G3 purified from the ascites fluids and used in all of the following immunoassays were found to be specific for the ORFV059 protein. Ig isotyping of the Mab A3 and G3 indicated that both were members of the IgG2b subclass that have the kappa light chain (data not shown).
Western blot analysis of the reactivity of ten potential anti-ORFV059 protein Mab with purified ORFV and rORFV059 protein. 2 μg of protein from purified ORFV-Jilin (a) or 1 μg rORFV059 (b) were resolved using SDS-PAGE, transferred to nitrocellulose paper, and probed with the Mab secreted by hybridomas A3–H5 (corresponding lanes A3–H5). The reactivity of sera from sheep diagnosed as having orf (lanes S1 and S2) against purified virus protein (a) was also tested. The positions of the protein molecular weight markers are indicated on the left-hand side of the figure while the location of an approximately 39 kDa protein corresponding in molecular weight to ORFV059 protein or rORFV059 is shown on the right-hand side of the figure
Immunoprecipitation of the ORFV059 protein with either Mab A3 or G3
To create a reagent for testing the capability of either Mab A3 or G3 to immunoprecipitate the ORFV059 protein, this virus polypeptide was synthesized in OFTu cells as a product fused to a Flag tag. Initially, to test the altered protein’s suitability as an antigen, lysates of OFTu cells transfected with the parental pCMVTag4A plasmid (expressing only the Flag tag) or the recombinant pCMV-ORFV059-Flag plasmid (expressing the ORFV059 protein-Flag tag fusion) were subjected to Western blotting using either Mab A3 or G3 (Fig. 4a, top panels) as well as an anti-GADPH antibody as a loading control (Fig. 4a, bottom panels). The presence of a Flag-associated epitope did not alter the specificity of either Mab as both only recognized an approximately 39 kDa protein (ORFV059Flag) in the lysates of the pCMV-ORFV059-Flag plasmid-transfected cells (Fig. 4a, top panels, lanes 2 and 4) and did not interact with any proteins including a different Flag-tagged moiety present in the lysates of the pCMVTag4A plasmid-transfected cells (Fig. 4a, top panels, lanes 1 and 3). To test whether Mab A3 or G3 also interact with the native ORFV059 protein, they were first incubated with lysates from the pCMV-ORFV059-Flag plasmid-transfected cells and then with protein G agarose beads before being subjected to centrifugation. Western blotting analysis of the proteins bound to the agarose beads with an anti-Flag antibody enabled detection of an approximately 39 kDa protein (ORFV059Flag) in either case (Fig. 4b, lanes 3, 4). As this band was conspicuously absent when a lysate from cells transfected with the control pCMVTag4A plasmid was exposed to Mab A3 (Fig. 4b, lane 1) or when a lysate from cells transfected with the ORFV059 protein-expressing, pCMV-ORFV059-Flag plasmid was not exposed to Mab A3 (Fig. 4b, lane 2), a specific interaction between Mab A3 and the ORFV059 protein was required for successful immunoprecipitation. Thus, Mab A3 and, most likely, Mab G3 recognize not only the SDS-denatured form of the ORFV059 protein but also its native, non-denatured one.
Western blot analysis of the reactivity of purified Mab A3 or G3 with lysates of transfected OFTu cells. OFTu cells were transfected with plasmid pCMVTag4A or pCMV-ORFV059Flag and were lysed 24 h later. a Proteins in the lysates from the pCMVTag4A (lanes 1 and 3) or pCMV-ORFV059Flag (lanes 2 and 4) transfected cells were resolved by using SDS-PAGE, transferred to nitrocellulose paper, and probed with purified Mab A3 (top panels, lanes 1 and 2), purified Mab G3 (top panels, lanes 3 and 4), or anti-GAPGH antibodies (bottom panels, lanes 1–4). The positions of the protein molecular weight markers are indicated on the left-hand side of the figure while the locations of the Flag-tagged ORFV059 protein (ORFV059Flag) and of GADPH are shown on the right-hand side of the figure. b Proteins in the lysates from the pCMVTag4A (lane 1) or pCMV-ORFV059Flag (lanes 3 and 4) transfected cells were incubated with purified Mab A3 (lanes 1 and 3) or G3 (lane 4) and the resultant complexes were immunoprecipitated with protein G agarose beads. Another aliquot of the lysate prepared from the pCMV-ORFV059Flag (lane 2) transfected cells (lane 2) was similarly treated except that the first incubation occurred in the absence of either Mab. Proteins in the precipitates were resolved using SDS-PAGE, transferred to nitrocellulose paper, and probed with an anti-Flag Mab. The positions of the protein molecular weight markers are indicated on the left-hand side of the figure while the locations of the Flag-tagged ORFV059 protein (ORFV059Flag) and of the light chain of IgG (IgG Light chain) are shown on the right-hand side of the figure
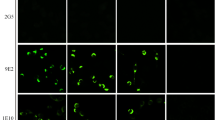
Immunoflourescent detection of the ORFV059 protein with Mab A3 or G3
To determine whether Mab A3 or G3 could be used for the immunodetection of the ORFV059 protein in virus-infected mammalian cells, monolayers of OFTu cells were infected with ORFV-Jilin and then fixed at various times p.i. The permeabilized cells were incubated with Mab A3 or G3 and then with goat-anti-mouse IgG antibodies conjugated to Alex Fluor 488 (Fig. 5, column ORFV059). To visualize the cell nucleus and virus factories, the cells were counterstained with DAPI (Fig. 5, DAPI columns, blue images). As seen in Fig. 5 (merge columns), Mab A3 was only reactive in virus-infected cells as evidenced by the presence of virus factories within them and that this reactivity was localized to these sites of virus DNA replication. Moreover, the associated fluorescence signal was only observed starting at 12 h p.i. Thus, as the presence of intracellular ORFV059 protein can be detected by using Mab A3, it would appear that this late-expressed protein is localized exclusively in virus factories. Similar results were obtained when Mab G3 replaced Mab A3 (data not shown).
Immunofluorescent detection of intracellular ORFV059 protein. OFTu cells were infected with ORFV-Jilin at a MOI of 5 and fixed with formaldehyde at the indicated times p.i. The fixed cells were probed with purified Mab A3, incubated with anti-mouse IgG antibodies conjugated to Alex Fluor 488, stained with DAPI, and then examined by using an inverted fluorescent microscope. As a point of reference, the bright field images (BF) of the cells are also shown in Fig. 5. Arrows and arrowheads show locations of the viral factories and cell nuclei, respectively. Bar = 10 μm
Expression of the ORFV059 gene during ORFV-Jilin infection
Based on the temporal-dependent, immunoflourescent detection of the ORFV059 protein in infected OFTu cells, the responsible gene is likely transcribed late during the virus’s replicative cycle. To confirm this prediction, the kinetics of ORFV059 gene transcription was compared to that of a known early gene (ORFV024) [26] in the absence and presence of AraC, an inhibitor of poxvirus DNA synthesis [25] (Fig. 6a). As expected, RT-PCR amplicons corresponding to a portion of the ORFV024 gene transcript were generated from RNA isolated from OFTu cells, irregardless of their exposure status to AraC, starting at 1 h p.i. In contrast, ORFV059 gene transcripts were only detected much later (12 h p.i.) and then only in those cells cultured in the absence of AraC. This apparently delayed expression of the OTFV ORFV059 gene was also manifested when infected cell lysates were subjected to Western blotting (Fig. 6b). In this case, although the housekeeping GAPDH protein was readily found in all samples, only those prepared at 12 and 24 h p.i. but not at 4 or 8 h p.i. contained the ORFV059 protein.
Temporal expression of the ORFV-Jilin ORFV024 and ORFV059 genes. a OFTu cells were infected with ORFV-Jilin at a MOI of 10 in the absence or presence of AraC and their RNA was isolated at the indicated times p.i. These RNAs were subjected to RT-PCR using primers specific for the amplification of cDNAs derived from transcripts encoding either ORFV024 or ORFV059 gene transcripts. The resultant amplicons were electrophoresed in agarose gels and visualized by staining with EtBr. The results shown are representative of three independent experiments. b OFTu cells were infected with ORFV-Jilin at a MOI of 10 and lysed at the indicated times p.i. and analyzed by SDS-PAGE. Proteins in the lysates were resolved using SDS-PAGE, transferred to nitrocellulose paper, and probed with purified Mab A3 (top panel) or anti-GAPGH antibodies (bottom panel). The positions of the 36 kDa protein molecular weight marker are indicated on the left-hand side of the figure while the locations of the ORFV059 and GADPH protein are shown on the right-hand side of the figure
Immunocytochemical examination of ORFV-Jilin particles with Mab A3 or G3
To establish whether either of the two anti-ORFV059 protein Mabs A3 and G3 recognized this entity as a component of a mature virion, interaction of either Mab with purified ORFV-Jilin virus was assessed by subsequent treatment of the Mab-exposed virus with goat anti-mouse IgG coupled to gold particles. Upon examination of the treated viruses by using an electron microscope, gold grains were found to be on or close to their outer membranes (Fig. 7a, b). Thus, both Mabs appeared to bind to an exposed portion of the ORFV059 protein present in mature virions.
Immunocytochemical detection of virus-associated ORFV059 protein. Mature ORFV-Jilin particles were purified by using sucrose gradient-ultracentrifugation, stained with purified Mab A3 a or G3 b and subsequently with an anti-mouse IgG antibody attached to gold grains, and then examined by using an electron microscope. Arrows indicate the locations of representative gold grains. Scale bars = 200 nm
Neutralization of ORFV-Jilin infectivity by Mab A3 or G3
As the ORFV059 protein is involved in virus adsorption to host cells [12, 13] and Mab A3 and G3 bind to this protein, either or both Mab could conceivably neutralize ORFV. To test this possibility, the relative infectivity of an ORFV-Jilin virus preparation after incubation with either antibody was measured in vitro using a modified fluorescent focus neutralization assay (Fig. 8). As compared to the decreased titer when normal mouse serum was used as a negative control reagent, statistically significant reductions in virus infectivity were observed after pre-exposure of the virus to either of the two lowest dilutions of either Mab. Thus, both Mab possess ORFV neutralizing capability.
Virus neutralization activity of anti-ORFV059 protein Mab A3 and G3. 100 TCID50 of ORFV-Jilin were incubated with the indicated dilutions of purified Mab A3, purified Mab G3, or normal mouse serum (NMS) before being used to infect monolayers of OFTu cells. After 24 h, the cells were fixed with formaldehyde and screened for the presence of ORFV using a modified fluorescent focus neutralization assay. Results are presented as the percent reduction in infectivity of the treated virus solutions as compared to the untreated one. Statistically significant differences (P < 0.001) between the percent reductions afforded to virus infectivity as a consequence of exposure to a specific dilution of Mab as compared to the corresponding dilution of NMS are indicted by an asterisks. The results from one of three independent experiments are presented
Immunohistochemical detection of ORFV-Jilin with Mab A3 or G3
Due to the successful detection of ORFV particles with Mab A3 or G3, these antibodies were used to determine the virus’s distribution in the skin lesion of a sheep afflicted with orf disease. Accordingly, sectioned tissue was subjected to an immunohistochemical process that independently utilized each Mab. As seen in Fig. 9a, c, the virus was prevalent in the basal and differentiated suprabasal keratinocytes in the epidermal layer and also in inflammatory cells that had infiltrated the subcutaneous tissue. In contrast, the virus appeared to be absent from the connective tissues in the subcutaneous region. It should be noted that the observed virus localizations appeared to be genuine as replacement of the Mab with normal mouse serum precluded any positive reactions in the tissue sections (Fig. 9b, d).
Immunohistochemical detection of ORFV-Jilin in an orf lesion. Tissue that originated from a sheep’s skin and encompassed an orf lesion was excised. Representative sections were consecutively incubated with hydrogen peroxide; purified Mab A3 a, purified Mab G3 c or normal mouse serum (b and d); anti-mouse IgG antibody conjugated to HRP; DAB; and hematoxylin. The stained sections were visualized with a light microscope at 400× magnification
Mab A3 and G3 reactivity with different orf parapoxvirus strains and other poxvirus
Reactivity of Mabs A3 and G3 with different orf parapoxvirus strains and poxvirus from other species was observed using ELISA and Immunohistochemical detection. As given in Table 2, Mab A3 and G3 can recognize both various ORFV strains isolated from China and American strain OV-IA82. No cross reactivity was detected with other poxvirus such as vaccinia virus, Goatpoxvirus and Fowlpoxvirus.
Discussion
The ability of a Mab to recognize the same protein produced by different virus strains is contingent on conservation of the amino acid sequence comprising the relevant epitope. In this regard, the previously reported variability of the deduced amino acid sequences of the ORFV059 protein associated with ORFV-NZ2, ORFV-OV/C2, ORFV-OV/7, ORFV-OV/20, ORFV-OV-IA82, ORFV-OV/Torino, ORFV-OV/mi-90 and ORFV-SA00 [17] as well as the two amino acid deletion in the ORFV-Jilin ORFV059 protein as compared to the ORFV-OV-IA82 homolog and the accompanying 10 amino acid substitutions described here might impact the effectiveness of a single, universal anti-ORFV059 protein Mab. Therefore to circumvent this potential problem, the ORFV-Jilin genomic DNA was used as a template to obtain an invariant ORFV059 gene for expression of rORFV059 protein. Although lysates prepared from mammalian cells infected with other ORFV strains have been used as an antigenic source to develop Mab against this protein [29, 30], we chose to initiate this process with the relevant protein that had been purified from a bacterial expression system in the absence of other ORFV proteins. As this second protocol can be completed in a shorter time and can utilize the recombinant protein for a simple identification of positive hybridomas [31, 32], we were easily able to obtain Mab that specifically recognized the ORFV-Jilin ORFV059 protein against a background of other potential viral and mammalian antigens. Reactivity of Mab A3 and G3 with different ORFV strains isolated from China and American strain OV-IA82 but not with other poxvirus indicates that Mab A3 and G3 can be used for orf diagnosis in China or other countries.
The ELISA and Western blot results conformed that there were no cross-reactivity between A3 or G3 and His tag. The subsequent experiments showed that prepared monoclonal antibodies can recognize well not only the SDS-denatured viral ORFV059 protein but also the intact non-denatured one by immunoprecipitation, immunofluorescence, and immunocytochemistry and immunohistological assays.
Our data demonstrate that ORFV059 is a late gene. The transcription kinetics shows that ORFV059 mRNA initiated at least 12 h p.i. (Fig. 6a). Late expression of ORFV 059 protein in ORFV infected OFTu cells was demonstrated by SDS-PAGE and Western blot analysis (Fig. 6b). A polypeptide about 39 kDa was detected at 12 h p.i., indicating that synthesis was initiated between 10 and 12 h p.i. Immunofluorescence assay also demonstrated that ORFV059 protein can be detected as early as 12 h p.i. (Fig. 7). Vaccinia virus H3L protein, ORFV059 homolog, is a late gene. The synthesis of H3L was initiated between 6 and 12 h p.i. H3L was detected at as early as 12 h p.i. by Western blot analysis and 8 h p.i. by immunofluorescence microscopy [33]. Neutralization experiments show that both A3 and G3 have neutralization activity (Fig. 8).
Orf is usually diagnosed based on pathological examinations and clinical signs [12], but in early times of the disease, it is difficult to recognize. Specific antibodies such as Mab have a wide range of potent applications in diagnostic and research [34]. As we showed in the Results, the prepared Mab can be used for diagnostic and epidemiological investigation using the ELISA, Western blot and immunohistological detection assays, for studying the mechanism of pathogenesis through detecting the virus entry and location using the immunoprecipitation, immunofluorescence, immunogold assays and immunohistochemistry [1–4, 12], and also for developing the possible subunit vaccine and therapeutic medicine [11, 35].
The results presented here demonstrated that the anti-ORFV059 monoclonal antibodies produced in this study are specific, and can be used as valid tools for further research on the mechanism of ORFV pathogenesis and development of the immunodiagnostic assay and vaccine to prevent and control the orf disease.
References
T. Vikøren, A. Lillehaug, J. Akerstedt, T. Bretten, M. Haugum, M. Tryland, Vet. Microbiol. 127, 10–20 (2008)
S. Al-Salam, N. Nowotny, M.R. Sohail, J. Kolodziejek, T.G. Berger, J. Cutan. Pathol. 35, 603–607 (2008)
M.C. McElroy, H.F. Bassett, Vet. J. 174, 663–664 (2007)
S.B. Fleming, I.E. Anderson, J. Thomson, D.L. Deane, C.J. McInnes, C.A. McCaughan, A.A. Mercer, D.M. Haig, J. Gen. Virol. 88, 1922–1927 (2007)
J. Guo, Z. Zhang, J.F. Edwards, R.W. Ermel, R.W. Ermel, C. Taylor Jr, A. de la Concha-Bermejillo, Virus Res. 93, 169–179 (2003)
D.M. Haig, C.J. McInnes, Virus Res. 88, 3–16 (2002)
D.L. Yirrell, H.W. Reid, M. Norval, G. Entrican, H.R. Mille, Vet. Immunol. Immunopathol. 28, 219–235 (1991)
D.G. Diel, S. Luo, G. Delhon, Y. Peng, E.F. Flores, D.L. Rock, J. Virol. 85, 264–275 (2011)
A.A. Mercer, N. Ueda, S.M. Friederichs, K. Hofmann, K.M. Fraser, T. Bateman, S.B. Fleming, Virus Res. 116, 146–158 (2006)
G. Delhon, E.R. Tulman, C.L. Afonso, Z. Lu, A. de la Concha-Bermejillo, H.D. Lehmkuhl, M.E. Piccone, G.F. Kutish, D.L. Rock, J. Virol. 78, 168–177 (2004)
A. Scagliarini, L. Gallina, F. Dal Pozzo, M. Battilani, S. Ciulli, S. Prosperi, Virus Res. 105, 107–112 (2004)
F.G. da Fonseca, E.J. Wolffe, A. Weisberg, B. Moss, J. Virol. 74, 7508–7517 (2000)
F.G. da Fonseca, E.J. Wolffe, A. Weisberg, B. Moss, J. Virol. 74, 7518–7528 (2000)
A. Scagliarini, S. Ciulli, M. Battilani, I. Jacoboni, F. Montesi, R. Casadio, S. Prosperi, Arch. Virol. 147, 1989–1995 (2002)
J.L. Tan, N. Ueda, A.A. Mercer, S.B. Fleming, J. Gen. Virol. 90, 614–625 (2009)
L. Gallina, A. Scagliarini, S. Ciulli, S. Prosperi, Vet. Res. Commun. 28(S1), 291–293 (2004)
K. Zhao, D. Song, W. He, H. Lu, B. Zhang, C. Li, K. Chen, F. Gao, Vet. Microbiol. 142, 408–415 (2010)
M. Zheng, Q. Liu, N. Jin, J. Guo, X. Huang, H. Li, W. Zhu, Y. Xiong, Mol. Cell. Probes 21, 276–281 (2007)
Y. Li, F. Wei, C. Chen, Y. Wang, H. Zhang, Y. Ren, Z. Guo, Chin. J. Vet. Sci. 31, 1270–1275 (2011)
W. Dong, M. Li, H. Bi, Y. Li, J. Wu, L. Qu, Int. J. Parasitol. 31, 57–62 (2001)
G. Wang, H. Sun, Y. Yu, Y. Wu, B. Lou, S. Liu, Sci. Agric. Sin. 42, 4077–4084 (2009)
W. Joklik, Virology 18, 9–18 (1962)
H.T. Zwartouw, J.C.N. Westwood, G. Appleyyard, J. Gen. Microbiol. 29, 523–529 (1962)
S. Luo, M. Ho, B.A. Wilson, Toxicon 51, 597–605 (2008)
L.R. Aminova, S. Luo, Y. Bannai, M. Ho, B.A. Wilson, Protein Sci. 17, 945–949 (2008)
Chisholm, S.E. Chisholm, H.T. Reyburn, J. Virol. 80, 2225–2233 (2006)
D.G. Diel, G. Delhon, S. Luo, E.F. Flores, D.L. Rock, J. Virol. 84, 3962–3973 (2010)
W.H. Wu, Y. Fang, R. Farwell, M. Steffen-Bien, R.R. Rowland, J. Christopher-Hennings, E.A. Nelson, Virology 287, 183–191 (2001)
S.L. Lard, J.T. Roehrig, L.D. Pearson, Vet. Immunol. Immunopathol. 28, 247–258 (1991)
C.P. Czerny, R. Waldmann, T. Scheubeck, Arch. Virol. 142, 807–821 (1997)
F.M. Housawi, G.M. Roberts, J.A. Gilray, I. Pow, H.W. Reid, P.F. Nettleton, K.J. Sumption, M.H. Hibma, A.A. Mercer, Arch. Virol. 143, 2289–2303 (1998)
M.D. Winther, G. Dougan, Biotechnol. Genet. Eng. Rev. 2, 1–39 (1984)
R.G. Werner, W. Berthold, H. Hoffmann, J. Walter, W. Werz, Biochem. Soc. Trans. 20, 221–226 (1992)
I. Lojkic, Z. Cac, A. Beck, T. Bedekovic, Z. Cvetnic, B. Sostaric, Virol. J. 7, 314 (2010)
M.R. Clark, Rev. Biol. Cel. 9, 1–79 (1986)
Acknowledgments
We thank Dr. Feng Gao (Jilin University) to provide us ORFV-Jilin isolate, Dr. Wenqi Dong (Southern Medical University) for vaccinia virus Tiantan strain, Dr. Sidang Liu (Shandong Agricultural University) for Fowlpoxvirus and Dr Qi Liu (Guangxi General Veterinary Agency) for Goatpoxvirus and ORFV GO-BT strain. We thank Dr. Daniel Rock (University of Illinois at Urbana-Champaign) allow us to test the cross reaction of A3 and G3 with OV-IA82. This study was partially supported by the grant (No. 31070138 and No. 31170147 to Luo Shuhong) from the National Natural Science Foundation of China (NSFC).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hong Li, Zhangyong Ning and Wenbo Hao contributed equally to this study.
Rights and permissions
About this article
Cite this article
Li, H., Ning, Z., Hao, W. et al. Identification and characterization of monoclonal antibodies against the ORFV059 protein encoded by Orf virus. Virus Genes 44, 429–440 (2012). https://doi.org/10.1007/s11262-011-0710-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-011-0710-9