Abstract
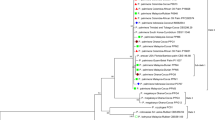
Rice tungro disease, one of the major constraints to rice production in South and Southeast Asia, is caused by a combination of two viruses: Rice tungro spherical virus (RTSV) and Rice tungro bacilliform virus (RTBV). The present study was undertaken to determine the genetic variation of RTSV population present in tungro endemic states of Indian subcontinent. Phylogenetic analysis based on coat protein sequences showed distinct divergence of Indian RTSV isolates into two groups; one consisted isolates from Hyderabad (Andhra Pradesh), Cuttack (Orissa), and Puducherry and another from West Bengal, Coimbatore (Tamil Nadu), and Kanyakumari (Tamil Nadu). The results obtained from phylogenetic study were further supported with the SNPs (single nucleotide polymorphism), INDELs (insertion and deletion) and evolutionary distance analysis. In addition, sequence difference count matrix revealed 2–68 nucleotides differences among all the Indian RTSV isolates taken in this study. However, at the protein level these differences were not significant as revealed by Ka/Ks ratio calculation. Sequence identity at nucleotide and amino acid level was 92–100% and 97–100%, respectively, among Indian isolates of RTSV. Understanding of the population structure of RTSV from tungro endemic regions of India would potentially provide insights into the molecular diversification of this virus.

Similar content being viewed by others
References
K. Muralidharan, D. Krishnaveni, N.V.L. Rajarajeshwari, A.S.R. Prasad, Curr Sci 85, 1143 (2003)
M. Periasamy, F.R. Niazi, V.G. Malathi, J. Virol. Methods 134, 230 (2006)
R. Hull, Annu. Rev. Phytopathol 34, 275 (1996)
H. Hibino, Plant Dis 67, 774 (1983)
M.C. Jones, K. Gough, I. Dasgupta, B.L. Subba Rao, J. Cliffe, R. Qu, P. Shen, M. Kaniewska, M. Blakebrough, J.W. Davies, R.N. Beachy, R. Hull, J. Gen. Virol 72, 757 (1991)
P. Shen, M. Kaniewska, C. Smith, R.N. Beachy, Virology 193, 621 (1993)
A. Druka, T. Burns, S. Zhang, R. Hull, J. Gen. Virol 77, 1975 (1996)
R. Joshi, V. Kumar, I. Dasgupta, J. Virol. Methods 109, 89 (2003)
A. Banerjee, S. Roy, J. Tarafdar, Virus Genes 43, 398 (2011)
A. Banerjee, S. Roy, J. Tarafdar, Virus Genes. (2011) doi:10.1007/s11262-011-0680-y
Y.P. Tian, J.L. Liu, X.Q. Yu, L.P. Lei, X.P. Zhu, J.P.T. Valkonen, X.D. Li, Arch. Virol. 152, 191 (2007)
J.D. Thompson, T.J. Gibson, F. Plewniak, F. Jeanmougin, D.G. Higgins, Nucleic Acids Res. 24, 4876 (1997)
T.A. Hall, Nucleic Acids Symp. Ser 41, 95 (1999)
K. Tamura, D. Peterson, N. Peterson, G. Stecher, M. Nei, S. Kumar, Mol. Biol. Evol 28, 2731 (2011)
O. Azzam, M. Arboleda, K.M. Umadhay, J.B. DeLos Reyes, F.S. Cruz, A. Mackenzie, K.L. McNally, Arch. Virol 145, 2657 (2000)
S.K. Mangrauthia, P. Malathi, S.M. Balachandran, C.S. Reddy, B.C. Viraktamath, J Plant Biochem Biotech 19, 263 (2010)
M.S. Rosenberg, BMC Bioinformatics 6, 102 (2005)
D. Krishnaveni, C.S. Reddy, G.S. Laha, C.N. Neeraja, G.S.V. Prasad, M.S. Prasad, S.K. Mangrauthia, K. Muralidharan, B.C. Viraktamath, Technical bulletin No. 43 Directorate of Rice Research (ICAR) Rajendranagar, Hyderabad, (2009)
Z. Fan, G. Dahal, I. Dasgupta, J. Hay, R. Hull, J. Gen. Virol 77, 847 (1996)
S. Zhang, J.W. Davies, R. Hull, Virus Genes 15, 61 (1997)
S. Zhang, G. Lee, J.W. Davies, R. Hull, Arch. Virol 142, 1873 (1997)
D. Posada, K.A. Crandall, E.C. Holmes, Annu. Rev. Genet 36, 75 (2002)
S.K. Mangrauthia, B. Parameswari, R.K. Jain, S. Praveen, Biochem. Genet 46, 835 (2008)
C. Roth, D.A. Liberles, BMC Plant Biol 6, 12 (2006)
L. Duret, Trends Genet 16, 287 (2000)
Acknowledgments
Authors are highly thankful to Dr. R.M. Sundaram (Senior Scientist, Directorate of Rice Research, Hyderabad) for providing the tungro infected rice samples of Puducherry. Financial support obtained under the SERC fast track scheme of Department of Science and Technology (DST), Govt. of India is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mangrauthia, S.K., Malathi, P., Agarwal, S. et al. Genetic variation of coat protein gene among the isolates of Rice tungro spherical virus from tungro-endemic states of the India. Virus Genes 44, 482–487 (2012). https://doi.org/10.1007/s11262-011-0708-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-011-0708-3