Abstract
The threat of highly virulent avian influenza, such as H5N1 and swine-origin H1N1 influenza viruses, bring out an urgent need to develop a universal influenza vaccine, which may provide cross-protection against different strain of influenza A viruses. The extra-domain of influenza M2 protein (M2e), which is almost completely conserved among all subtypes of influenza A viruses, is considered as a promising candidate target for the development of a broad-spectrum recombinant influenza A vaccine. The results of several preclinical studies with M2e protein, with or without carriers, have already proved the successful protection of M2e-based vaccinated animal model against lethal challenge of heterologous and homologous influenza A viruses. Recently, the results of Phase I/II clinical trail studies with M2e-based vaccines have raised hopes for considering these vaccines against seasonal and pandemic influenza A strains. Hence, it is expected that more and more effective and safe universal influenza vaccines based on M2e will be developed for prevention of seasonal and pandemic influenza in the near future.
Similar content being viewed by others
Introduction
Yearly development of influenza vaccines that are antigenically matched to circulating strains poses extraordinary challenges. A rapidly developing pandemic would shorten the time for strain identification and vaccine preparation; meanwhile, antigenic changes would continue. Moreover, the need to immunize an entirely naive population would exacerbate problems with vaccine production and supply.
Emerging and re-emerging infectious diseases by influenza A virus in humans and animals have been reported with increased frequency in recent years [1]. The emergence of high-pathogenicity avian influenza (HPAI) viruses in domestic poultry and the increasing number of cases of direct transmission of avian influenza viruses of different subtypes to humans are a significant threat to public health because of the potential for pandemic spread of these viruses [2–4].
The recently emerged swine-origin H1N1 influenza viruses are being detected in an increasing number of countries, and their global spread have resulted in a considerable number of infected individuals [5, 6].
The threat of highly virulent avian influenza, such as H5N1 and swine-origin H1N1 viruses, brings out an urgent need to develop a universal influenza vaccine, which could provide cross-protection against different influenza virus strains. Therefore, with the continuous threat of an influenza pandemic, possibly arising from animal reservoirs, such as pigs and avian, there is an urgent need to develop safe and effective vaccines against divergent influenza A virus, particularly the current epidemic strains of swine-origin H1N1 and the highly pathogenic H5N1 viruses.
Recently, the attentions of scientists and pharmacologists have focused on the M2 protein. New vaccines and drugs directed against M2 protein should be more universal than currently used one.
Therefore, in this article, we have reviewed the importance, molecular structure and function of the influenza A virus M2 protein, which remains nearly invariable since the 1918 Spanish flu [7], as a universal vaccine candidate, and then discussed the roles of M2e-specific antibody in reducing the rate of viral replication and viral shedding in influenza infected hosts. Finally, we have also summarized some preclinical and clinical studies with M2e-vaccines, which are underway toward validation of its protective efficacy in human.
Influenza viruses and key proteins
Influenza virus is a respiratory pathogen belonging to the family of the Orthomyxoviridae. There are three types of influenza (A, B, C) distinguished by the antigenic differences in the major internal proteins of the virus, i.e., nucleoprotein (NP) and matrix protein (M1). Meanwhile, Influenza A viruses can be divided into subtypes on the basis of genetic and antigenic differences in their main surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA) [8]. So far, 16 HA (H1–H16) and 9 NA (N1–N9) glycoprotein subtypes have been identified in influenza A viruses [8, 9].
All influenza A and B viruses possess eight RNA segments that encode 11 viral proteins (Fig. 1), whereas influenza C viruses only have seven RNAs that encode 9 viral proteins [8, 10]. Each segment contains a coding region that encodes one or two proteins. Three segments encode proteins that form the virus polymerase complex: basic polymerase 2 (PB2; 2,277 nucleotides in the protein-coding region, in segment 1); basic polymerase 1 (PB1; 2,271 nucleotides, segment 2 and also encodes a small proapoptotic mitochondrial protein that is translated in a different reading frame—PB1-F2); and the acidic protein (PA; 2,148 nucleotides, segment 3). Two segments encode surface envelope glycoproteins that function as viral antigens: HA (1,698 nucleotides, segment 4), which is divided into two domains (or subunits)—HA1 and HA2—and NA (1,407 nucleotides, segment 6). A single segment encodes a NP (1,494 nucleotides, segment 5). The seventh segment encodes two proteins that share a short overlapping region: M1 (756 nucleotides) encodes the main component of the viral capsid, and M2 (291 nucleotides), which is an integral membrane protein, functions as an ion channel. Segment 8 encodes a nonstructural protein, NS1 (690 nucleotides), and NS2 protein (363 nucleotides) [11, 12].
Schematic representation of influenza A virus. The influenza A virus particle has a lipid envelope that is derived from the host cell membrane. Three envelope proteins including: hemagglutinin (HA), neuraminidase (NA), and an ion channel protein (matrix protein 2, M2) are embedded in the lipid bilayer of the viral envelope. HA (rod shaped) and NA (mushroom shaped) are the main surface glycoproteins of influenza A viruses. The ribonucleoprotein complex comprises a viral RNA segment associated with the nucleoprotein (NP) and three polymerase proteins (PA, PB1, and PB2). The matrix (M1) protein is associated with both ribonucleoprotein and the viral envelope. A small amount of nonstructural protein 2 is also present, but its location within the virion is unknown. Figure was derived from previously published paper [2]
Types of influenza vaccines and their limitation
Current influenza virus vaccines attempt to induce strong antibody responses against the viral glycoproteins HA and, with lesser emphasis, NA because their protective efficacy is well documented. Thus, typical HA-specific antibodies neutralize viral infectivity and fully protect against infection when they are present at sufficient concentration in the lining fluid of the respiratory tract, and typical NA-specific antibodies inhibit the release of newly formed virus from infected host cells and thus limit the spread and shedding of virus during infection [13].
The currently developed influenza vaccines mainly contain three subtypes of influenza virus which are selected by the WHO based on global surveillance of influenza viruses by references laboratories from the strains predicted to circulate in the upcoming year [14, 15].
Theses vaccines are trivalent: containing two subtypes of influenza A and one of influenza B virus, that are using in the world under the different forms of: “Inactivated whole virion” vaccine which prepared by treating whole virus particles derived from virus-infected chicken eggs or mammalian cell lines with formalin or β-propiolactone; “Split virion, inactivated” or “disrupted virus” vaccines containing virus components prepared by treating whole viruses with detergents or organic solvents; “Surface antigen, inactivated” vaccines containing highly purified HA and NA antigens; “Surface antigen, inactivated, virosome” vaccines containing highly purified HA and NA antigens prepared from disrupted virus particles reconstituted into virosomes with phospholipids [6, 16, 17]. Live attenuated influenza vaccines are not available in all countries, but countries like Russia, Canada, and USA use it under some condition [18, 19].
These existing vaccines have a number of limitations, including: requirements of vaccine strains matching prevailing epidemic strain, long and complicated production cycle, limited production capacity, and potential to induce allergic responses in individuals who are allergy to egg products [20, 21]. Hence, the cumulative limitations urgently call for the development of novel universal influenza vaccines that might provide the required cross-clade protective immunity. Hence, in view of the inherent limitations in the approved, conventional influenza vaccines, the possibility of developing a universal influenza vaccine based on M2e antigen has attracted the attention of many researchers.
Function and molecular structure of M2 protein
The M2-protein was originally discovered by Lamb et al. [22]; it is produced by translation from a spliced mRNA derived from influenza gene segment 7, which also codes for the matrix protein M1 (Fig. 2).
The M2 protein is a type III integral membrane protein (nomenclature of von Heijne) [23]. It forms a proton-selective ion channel [24], which is activated at acidic pH [25, 26] and is a specific target of the anti-influenza drugs amantadine and rimantadine. These inhibitors affect two steps in the life cycle: virus uncoating and virus maturation. During virus entry, via receptor mediated endocytosis, M2 transport protons across the virus membrane reducing the pH of the virion interior. The low pH facilitates protein–protein dissociation between the matrix protein and the RNP (Fig. 3). During infection by the highly pathogenic influenza A virus subtypes H5 and H7, M2 ion channel increases the pH of the trans-Golgi network [28–30]. This is important to prevent premature acid activation of newly synthesized HA, which is cleaved intracellularly, and consequent inactivation of progeny virus [28, 29, 31]. The M2 also plays an important role in virus morphogenesis [30] and assembly [32].
Schematic representation of M2 protein role in fusion and uncoating process. M2 is a so-called viroporin with pH-controlled, passive proton-selective channel activity. Following endocytosis, this activity of M2 promotes acidification of the virion interior, a process that unties the interactions between the viral ribonucleoprotein complexes and the matrix and allows the entry of the viral core constituents into the cytosol [27]
The M2 protein has 96 amino acids, with three structural domains: an amino-terminal extracellular domain (comprising 23 residues), a transmembrane domain (TM) (19 residues), and a cytoplasmic domain (54 residues). The protein is phosphorylated mainly at Ser 64 [33] and contains palmitic acid covalently attached to a cytoplasmic cysteine residue [31]. The native form of the protein is disulfide-link homotetramer [34, 35]. Large numbers of M2 molecules are present on infected cell surfaces, with a ratio approximately two M2 molecules per HA trimer in CV-1 cells [36], but only a few (~23–60) M2 molecules are estimated to be incorporated into each virion [37].
A study in 2005 analyzed the variability of 24 amino acids in the M2 extracellular region (M2e) of 716 influenza A viruses grouped according to host restriction (human, avian, swine, equine, and other hosts) and found that 17 of the 24 amino acids were conserved at a rate of over 94% [7, 38] (Table 1). However, the N-terminal epitope SLLTEVET (residues 2–9) found to be conserved at a rate of over 99.3% among all subtypes of influenza A viruses and 100% among isolates from humans [7, 38]. Five amino acids, which are highlighted by bold letters, within the region of amino acids 10–20 of the M2e protein were shown to be host restricted: PIRNEWGCRCN (aa 10–20, human specific M2e sequence), PTRNGWECKCS (aa 10–20, avian), and PIRNGWECRCN (aa 10–20, swine) [38]. The low degree of variation in the M2 protein as compared to the HA and NA protein is clearly attributed to the genetic relation with M1 which is the most conserved protein of the virus (Fig. 2).
Roles of anti-M2e antibodies in preventing influenza infection
The M2 protein is present on the surface of the virus particles, where shielding by the larger and more abundant HA and NA proteins prevent it from in reacting efficiently with immune effector’s cells, but fairly abundant on virus-infected cells [36].
M2e, a small peptide, is a very poor immunogen in its native form and only a fraction of infected people develop anti-M2e antibodies, and there is no evidence that these low titer antibodies play a role in protection against a new infection by influenza A virus subtypes [39]. Reference [40] also showed that anti-M2 IgG titers in the blood raised after infection in M2e-based vaccinated mice, while mice infected with influenza virus did not generate sizeable titers of antibodies recognizing M2.
In mice immunized with inactivated virus, anti-HA antibodies have been shown to bind to free virus as well as to virus-infected cells. HA antibodies are more effective than M2e-antibodies, because they can (1) neutralize and prevent infection, (2) induce elimination of virus by Fc receptor-mediated phagocytosis [41], and (3) mediate killing of infected cells via antigen-dependent cell cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) [40].
In contrast to the HA protein, the M2e protein is not accessible to antibody and therefore antibodies to M2e are not virus-neutralizing [41]. However, M2e-specific antibodies bind strongly to virus infected cells and the protective antibodies act via antibody dependent cell cytotoxicity [42], and mediate the killing of infected cells by complement or cells of the innate immune system, natural killer (NK) cells [43]. In addition, antibodies specific for M2e bind virus to the cell and prevent the release of viral particles into the extracellular fluid or enhance the uptake by phagocytic cells via the Fc receptor [44]. The mechanism of protection by the antibodies induced by M2e vaccination has not yet been fully elucidated. Undoubtedly, ADCC or CDC plays an important role [40], but other protective mechanisms may also be involved.
Strategies to improve potency of the M2e protein
One of the concerns about influenza A vaccine based on M2e protein is their limited potency; hence, optimal approaches to enhance immunogenicity of M2e protein immunization remain to be established. It seems by linking this small and low immunogen M2e protein to an appropriate carrier we can render it very immunogenic.
Therefore, some universal influenza vaccines have been designed by fusing M2e with some components to facilitate the formation and maintenance of tetrameric structure for improving the immunogenicity of M2e. For instance, fusion of a tetrameric domain of GCN4 (General Control Nondepressible 4), which is a eukaryotic transcriptional activator protein, to M2e resulted in a recombinant tetrameric protein, M2e-tGCN4, developed potent M2e-specific antibody responses, which fully protected the vaccinated mice from lethal challenge of mouse-adapted influenza A virus [45].
The M2e-hepatitis B core (M2e-HBc) fusion particles protected the vaccinated mice in challenge experiments by inducing M2e-specific antibodies [46].
The M2e was fused to the N-terminus of the truncated HSP70 molecule of Mycobacterium tuberculosis, HSP70359–610, and then E. coli-expressed [47] and P. pastoris-expressed [48] of this fusion proteins could significantly improve the immunogenicity of M2e protein (Ebrahimi et al., unpublished data).
A recombinant protein containing toll-like receptor 5 (TLR5) ligand salmonella typhimurium flagellin type 2 (STF2) fused to four tandem copies of conserved M2e, designated STF2.4xM2e, developed potent M2e-specific antibody responses and protected mice from a lethal influenza A virus challenge [49].
M2e virus-like particles (VLPs), including M2e displayed on papaya mosaic virus, human papillomavirus and phage Qβ-derived VLPs, have induced M2e-specific antibodies successfully, in which mice were protected against lethal challenge of influenza A virus [50, 51]. Other M2e fusion constructs that were shown to provide protection from influenza A virus challenge in mouse models include M2e-linkage to keyhole limpet hemocyanin (KLH), Neisseria meningitides outer membrane complex (OMPC), bovine serum albumin, glutathione transferase, a synthetic multiple antigen peptide, flagellin and cholera toxin-derived CTA1-DD [49, 52–59].
Review of attempts for M2e protein as a universal vaccine
Several M2e-based vaccines, as some mentioned in before section, have already been proved to provide a successful protection against the challenges with homologous and heterologous influenza viruses and among them some evidenced for the important protective role of M2e-specific antibodies.
The possibility that the M2e protein may serve as a vaccine target was first implied by Treanor et al. [60] in a study showed that passive transfer of M2 monoclonal antibody “14C2” in mice was able to accelerate lung viral clearance following a sublethal influenza A virus challenge. Slepushkin et al. [61] subsequently demonstrated that immunization with baculovirus-expressed recombinant full length M2 protein was able to shorten the duration of viral shedding and protect mice from lethal challenge.
Studies with different influenza A subtypes have shown that antibodies against the M2e protein inhibited virus replication in MDCK cells [37, 53] and also decreased the viral load in mice lungs and protected them from lethal challenge of influenza A viruses [54, 57, 61–63].
Passively transferred antibodies to the M2e protein fused with hepatitis B virus core (HBc) protein demonstrated 90–100% protection against lethal challenge with influenza A virus in mice [64].
However, the best protection was reported for mice vaccinated by the intranasal route with an M2e-HBc fusion protein construct and detoxified heat-labile E. coli enterotoxin adjuvant; almost none of these mice died after a virus challenge that killed 90% of control mice [65].
Generally, all existing experiments on M2e protein, with or without carriers, as a universal vaccine have been carried out mostly in mice and also some experiments in other species like ferrets, pigs, and monkey have shown that M2e-specific antibodies restricted subsequent virus replication and reduced illness and rate of mortality [54, 57, 61, 65, 66]. So, our understanding of immunology to influenza M2e protein is largely based on experiments in mice, and it is hard to find an example of a foreign protein which is immunogenic in the mouse but not in human.
On the contrary, vaccination of pigs with M2e-HBc fusion protein did not protect them against lethal challenge of swine influenza virus (SIV), H1N1, and the vaccinated pigs showed more sever clinical signs after challenge than the control pigs, this result did not agree with the results of previous study in which mice were partially protected after immunization with the same material [67]. Kitikoon et al. [68] in a recent study showed that M2e-vaccinated pigs that were challenged with swine influenza viruses, H1N1 and H1N2, not only were protected from lethal challenge of SIV infection, but the M2e vaccine reduced macroscopic lung lesions compared to the lesions in the nonvaccinated pigs. The difference between these two acquired results, the exact explanation for these differences remain unknown yet, has presented the complexity of immune phenomena in animal model and also persuaded the researcher to further relevant studies in pigs using influenza M2e-based vaccines.
Since the pig is a natural host of influenza virus, the swine influenza model seems a better model than the mouse model to study vaccination strategies not only for pigs, but also for humans. With this in mind, acquiring a good protection in pigs does not guarantee protection in humans.
Hence, Sanofi Pasteur Biologics Co., formerly Acambis (Massachusetts, USA) in its Phase I trial study in human has tested the safety and immunogenicity of a recombinant vaccine candidate (ACAM-FLU-A) consisting of the influenza A M2e domain, M2e-HBc fusion protein. The vaccine was shown to be immunogenic and well-tolerated, with no serious side-effects [69–71]. ACAM-FLU-A also protected ferrets from a deadly infection by the highly lethal avian H5N1 influenza strain “Vietnam 2004”, in which 70% of the vaccinated animals survived, while all the placebo-treated animals succumbed to the viral infection [69, 71].
VaxInnate Corp. (New Jersey, USA) in its first Phase I clinical trial study announced that M2e universal influenza vaccine candidate, an M2e-flagellin fusion influenza vaccine, with the 0.3 and 1.0 μg doses were safe and well tolerated in all healthy young volunteers, aged 18–49, and immunogenic in 75% vaccinees after the first dose and 96% after the second dose; moreover, VaxInnate is on track to begin a Phase II study of its M2e universal influenza vaccine candidate [72].
Although the results of these Phase I/II clinical trials have not been reported yet in the scientific literature, but these results have raised hopes for a universal influenza vaccine that could provide protection against seasonal and pandemic influenza strains.
Cytos Biotechnology (Schlieren, Switzerland), Pevion Biotech Ltd. (Bern, Switzerland), Dynavax Technologies Corp. (California, USA) and Merck & Co Inc. (New Jersey, USA) are also developing universal influenza vaccines based on: M2e-AP205 VLPs, M2e-Virosome, M2e-Iss or NP-M2e, M2e-KLH or M2e-OMPC fusion proteins, respectively [56, 63, 73, 74].
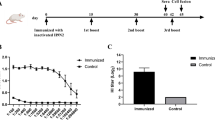
Recently, our scientific group in Razi vaccine and serum research institute, RVSRI (Tehran, Iran) has started basic and experimental studies on the influenza A virus M2e protein as a candidate for universal influenza vaccine in the forms of recombinant full length M2 protein [75, 76], recombinant fusion protein, designated M2e-HSP70359–610 [47, 48, 77], DNA vaccine (Ebrahimi et al., unpublished data), and VLPs (Ebrahimi et al., unpublished data). Accordingly, the relevant preclinical studies of recombinants M2e-HSP70359-610 fusion protein and full length M2 protein in C57BL/6 mice showed that linkage of M2e to C-terminal 28-kDa domain of Mycobacterium tuberculosis heat shock protein 70 (HSP70359–610) significantly increased the potency of this vaccine candidate based on M2e-HSP70 fusion protein (SME-FLU-A) and fully protected (100%) vaccinated mice (C57BL/6) from lethal challenge (100 LD90) of mouse-adapted influenza A virus, A/PR8/34 (H1N1), by significant inducing of M2e-specific antibody compared to the control groups of mice immunized with the recombinant full length M2 protein [75], influvac® vaccine 2009/2010 (Solvay, Netherlands), inactivated influenza vaccine 2009/2010 (Split Virion) BP (Sanofi Pateur MSD, UK), and PBS with the survival rates of 10, 40, 15, and 0%; respectively (Ebrahimi et al., unpublished data). Now, Razi institute is on track to begin an expanded Phase I clinical trails to evaluate the level of immunogenicity, antigen titration and side-effects of this recombinant fusion protein. Furthermore, the preclinical studies of M2e-based DNA and M2e-VLPs vaccines are carrying out in mice by our scientific group.
Conclusion
Influenza remains a serious disease despite the availability of antiviral drugs and inactivated trivalent vaccines. Antiviral drugs may not be in sufficient supply and the virus may acquire resistance to the available antiviral drugs. On the other hand, the production of a vaccine to a newly emerging strain would take 3–6 months, during which time a virus could spread globally and substantially cause problem on health care systems and the global economy. Hence, much research has been focused on alternative vaccine production systems including: universal vaccines based on M2e; plasmid-based reverse genetic systems for generating reference viruses; virus-like particles expressing the HA and NA proteins, some in combination with the M1 and M2 proteins; DNA vaccines, recombinant subunit vaccines, and peptide-based vaccines.
Basing on all studies reported to date, the M2e-based vaccines have been successfully tested in mice and other laboratory animals with fully protection, without side effects. In addition, the phase I trial results in human demonstrated that M2e-based vaccine as a recombinant influenza universal vaccines candidate are safe, well tolerated and immunogenic with no significant side-effects. Therefore, M2e is one of the most discussed new approaches for universal influenza vaccination. It is expected that more and more effective and safe universal influenza vaccines based on M2e will be developed for prevention of seasonal and pandemic influenza in the near future.
References
D.M. Morens, G.K. Folkers, A.S. Fauci, Nature 430, 242–249 (2004)
T. Horimoto, Y. Kawaoka, Nat. Rev. Microbiol. 3, 591–600 (2005)
B.C. Buxton, J.M. Katz, W.H. Seto, P.K. Chan, D. Tsang, W. Ho, K.H. Mak, W. Lim, J.S. Tam, M. Clarke, J. Infect. Dis. 181, 344–348 (2000)
M. Peiris, K.Y. Yuen, C.W. Leung, K.H. Chan, P.L. Ip, R.W. Lai, W.K. Orr, K.F. Shortridge, Lancet 354, 916–917 (1999)
R.J. Garten, C.T. Davis, C.A. Russell, B. Shu, S. Lindstrom, A. Balish, W.M. Sessions, X. Xu, E. Skepner, V. Deyde, Science 325, 197–201 (2009)
G. Neumann, T. Noda, Y. Kawaoka, Nature 459, 931–939 (2009)
W. Fiers, M.D. Filette, A. Birket, S. Neirynck, W.M. Jou, Virus Res. 103, 173–176 (2004)
P.F. Wright, R.G. Webster, in Fields Virology, ed. by D.M. Knipe, P.M. Howley (Lippincott Williams & Wilkins, Philadelphia, 2001), pp. 1533–1579
R.A. Fouchier, V. Munster, A. Wallensten, T.M. Bestebroer, S. Herfst, D. Smith, G.F. Rimmelzwaan, B. Olsen, A.D. Osterhaus, J. Virol. 79, 2814–2822 (2005)
P.C. Crawford, E.J. Dubovi, W.L. Castleman et al., Science 310, 482–485 (2005)
D.A. Steinhauer, J.J. Skehel, Genetics of influenza viruses. Ann. Rev. Genet. 36, 305–332 (2002)
R.A. Lamb, R.M. Klug, in Fundamental Virology, ed. by B.N. Fields, D.M. Knipe, P.M. Howley (Lippincott-Raven, Philadelphia, 2006), pp. 605–647
W. Gerhard, K. Mozdzanowska, D. Zharikova, Emerg. Infect. Dis. 12, 569–574 (2006)
Influenza health topic. www.who.int/topics/influenza/en/
D.M. Skowronski, C. Masaro, T.L. Kwindt, A. Mak, M. Petric, Y. Li, Vaccine 25, 2842–2851 (2007)
J.S. Oxford, C. Manuguerra, O. Kistner, A. Linde, M. Kunze, W. Lange, B. Schweiger, G. Spala, H.R. De Andrade, P.R. Perez Brena, Vaccine 23, 5440–5449 (2005)
K.L. Nichol, J.J. Treanor, J. Infect. Dis. 194, 111–118 (2006)
R. Belshe, M.S. Lee, R.E. Walker, J. Stoddard, P.M. Mendelman, Expert Rev. Vaccines 3, 643–654 (2004)
L.G. Rudenko, N.I. Lonskaya, A.I. Klimov, R.I. Vasilieva, A. Ramirez, Bull. WHO 74, 77–78 (1996)
B.E. Johansson, I.C. Brett, Vaccine 25, 3062–3065 (2007)
L. Widjaja, N. Ilyushina, R.G. Webster, R.J. Webby, Virology 350, 137–145 (2006)
R.A. Lamb, C.J. Lai, P.W. Choppin, Proc. Natl. Acad. Sci. USA 78, 4170–4174 (1981)
G.V. Heijne, EMBO J. 5, 3021–3027 (1986)
L.H. Pinto, L.J. Holsinger, R.A. Lamb, Cell 69, 517–528 (1992)
I.V. Chizhmakov, F.M. Geraghty, D.C. Ogden, A. Hayhurst, M. Antoniou, A.J. Hay, J. Physiol. 494, 329–336 (1996)
J.A. Mould, J.E. Drury, S.M. Frings, U.B. Kaupp, A. Pekosz, R.A. Lamb, L.H. Pinto, J. Biol. Chem. 275, 31038–31050 (2000)
http://www.microbiologybytes.com/virology/Orthomyxoviruses.html. Accessed June 2010
F. Ciampor, P.M. Bayley, M.V. Nermut, E.M. Hirst, R.J. Sugrue, A.J. Hay, Virology 188, 14–24 (1992)
S. Grambas, A.J. Hay, Virology 190, 11–18 (1992)
K. Iwatsuki-Horimoto, T. Horimoto, T. Noda, M. Kiso, J. Maeda, S. Watanabe, Y. Muramoto, K. Fujii, Y. Kawaoka, J. Virol. 80, 5233–5240 (2006)
R.J. Sugrue, R.B. Belshe, A.J. Hay, Virology 179, 51–56 (1990)
M.F. Mc Cown, A. Pekosz, J. Virol. 80, 8178–8189 (2006)
L.J. Holsinger, M.A. Shaughnessy, A. Micko, J. Virol. 69, 1219–1225 (1995)
L.J. Holsinger, R.A. Lamb, Virology 183, 32–43 (1991)
R.J. Sugrue, A.J. Hay, Virology 180, 617–624 (1991)
R.A. Lamb, S.L. Zebedee, C.D. Richardson, Cell 40, 627–633 (1985)
S.L. Zebedee, R.A. Lamb, J. Virol. 62, 2762–2772 (1988)
W. Liu, P. Zou, J. Ding, Y. Lu, Y.H. Chen, Microbes Infect. 7, 171–177 (2005)
R.A. Black, P.A. Rota, N. Gorodkova, H.D. Klenk, A.P. Kendal, J. Gen. Virol. 74, 143–146 (1993)
A. Jegerlehner, N. Schmitz, T. Storni, M.F. Bachmann, J. Immunol. 172, 5598–5605 (2004)
V.C. Huber, J.M. Lynch, D.J. Bucher, J. Le, D.W. Metzger, J. Immunol. 166, 7381–7388 (2001)
A.M. Eriksson, K.M. Schon, N.Y. Lycke, J. Immunol. 173, 3310–3319 (2004)
K. Mozdzanowska, K. Maiese, M. Furchner, W. Gerhard, Virology 254, 138–146 (1999)
K. Subbarao, T. Joseph, Nat. Rev. Microbiol. 7, 267–278 (2007)
F.M. De, W. Martens, K. Roose, T. Deroo, F. Vervalle, M. Bentahir, J. Vandekerckhove, W. Fiers, X. Saelens, J. Biol. Chem. 283, 11382–11387 (2008)
M. De Filette, W. Martens, A. Smet, M. Schotsaert, A. Birkett, P. Londoño-Arcila, W. Fiers, X. Saelens, Vaccine 26, 6503–6507 (2008)
S.M. Ebrahimi, M. Tebianian, Mol. Biol. Rep. 37, 2877–2883 (2010)
S.M. Ebrahimi, M. Tebianian, H. Toghyani, A. Memarnejadian, H.R. Attaran, Protein Expr. Purif. 70, 7–12 (2010)
J.W. Huleatt, V. Nakaar, P. Desai, Y. Huang, D. Hewitt, A. Jacobs, Vaccine 26, 201–214 (2008)
J. Bessa, N. Schmitz, H.J. Hinton, K. Schwarz, A. Jegerlehner, M.F. Bachmann, Eur. J. Immunol. 38, 114–126 (2008)
R.M. Ionescu, C.T. Przysiecki, X. Liang, V.M. Garsky, J. Fan, B. Wang, R. Troutman, Y. Rippeon, E. Flanagan, J. Shiver, J. Pharm. Sci. 95, 70–79 (2006)
S.M. Tompkins, Z.S. Zhao, C.Y. Lo, J.A. Misplon, T. Liu, Z. Ye, R.J. Hogan, Z. Wu, K.A. Benton, T.M. Tumpey, S.L. Epstein, Emerg. Infect. Dis. 13, 426–435 (2007)
W. Liu, H. Li, Y.H. Chen, F.E.M.S. Immunol, Med. Microbiol. 35, 141–146 (2003)
K. Mozdzanowska, J. Feng, M. Eid, G. Kragol, M. Cudic, L. Otvos, W. Gerhard, Vaccine 21, 2616–2626 (2003)
W.A. Ernst, H.J. Kim, T.M. Tumpey, A.D. Jansen, W. Tai, D.V. Cramer, J.P. Adler-Moore, G. Fujii, Vaccine 24, 5158–5168 (2006)
J. Fan, X. Liang, M.S. Horton, H.C. Perry, M.P. Citron, G.J. Heidecker, T.M. Fu, J. Joyce, C.T. Przysiecki, P.M. Keller, Vaccine 22, 2993–3003 (2004)
A.M. Frace, A.I. Klimov, T. Rowe, R.A. Black, J.M. Katz, Vaccine 17, 2237–2244 (1999)
W. Liu, Z. Peng, Z. Liu, Y. Lu, J. Ding, Y.H. Chen, Vaccine 23, 366–371 (2004)
K. Mozdzanowska, M. Furchner, D. Zharikova, J. Feng, W. Gerhard, J. Virol. 79, 5943–5951 (2005)
J.J. Treanor, E.L. Tierney, S.L. Zebedee, R.A. Lamb, B.R. Murphy, J. Virol. 64, 1375–1377 (1990)
V.A. Slepushkin, J.M. Katz, R.A. Black, W.C. Gamble, P.A. Rota, N.J. Cox, Vaccine 13, 1399–1402 (1995)
G. Zhao, Y. Lin, L. Du, J. Guan, S. Sun, H. Sui, Virol. J. 7, 9 (2010)
A.C. Tissot, R. Renhofa, N. Schmitz, I. Cielens, E. Meijerink, V. Ose, G.T. Jennings, P. Saudan, P. Pumpens, M.F. Bachmann, PLoS One 5, e9809 (2010)
S. Neirynck, T. Deroo, X. Saelens, P. Vanlandschoot, W.M. Jou, W. Fiers, Nat. Med. 5, 1157–1163 (1999)
M. De Filette, W. Min Jou, A. Birkett, K. Lyons, B. Schultz, A. Tonkyro, A. Resch, S. Fiers, Virology 337, 149–161 (2005)
T.M. Fu, K.M. Grimm, M.P. Citron, D.C. Freed, J. Fan, P.M. Keller, J.W. Shiver, X. Liang, J.G. Joyce, Vaccine 27, 1440–1447 (2009)
P.P. Heinen, F.A. Rijsewijk, E.A. De Boer-Luijtze, A.T. Bianchi, J. Gen. Virol. 83, 1851–1859 (2002)
P. Kitikoon, A.L. Vincent, B.H. Janke, B. Erickson, E.L. Strait, S. Yu, M.R. Gramer, E.L. Thacker, Vaccine 28, 523–531 (2010)
http://www.bioportfolio.com/biotech_news/acambis_3.htm. Accessed October 2009
W. Fiers, M. De Filette, K.E. Bakkouri, B. Schepens, K. Roose, M. Schotsaert, A. Birkett, X. Saelens, Vaccine 27, 6280–6283 (2009)
http://www.sciencedaily.com/releases/2008/01/080124185522.htm. Accessed July 2010
http://www.vaxinnate.com/pages/pressreleases/20081026_001.html. Accessed June 2010
http://science.thomsonreuters.com.cn/media/TR_DiseaseBriefing_H1N1_2009.pdf. Accessed April 2009
M. Schotsaert, M. De Filette, W. Fiers, Vaccines 8, 499–508 (2009)
S.M. Ebrahimi, M. Tebianian, K. Aghaiypour, H. Nili, A. Mirjalili, Mol. Biol. Rep. 37, 2909–2914 (2010)
S.M. Ebrahimi, K. Aghaiypour, H. Nili, Iran J. Biotechnol. 6, 229–232 (2008)
S.M. Ebrahimi, M. Tebianian, A. Mirjalili, H. Paykari, H.R. Varshovi, H. Toghyani, S. Moradi Bidhendi, H.R. Attaran, Arch. Razi Ins. 64, 71–76 (2009)
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11262-011-0598-4
Rights and permissions
About this article
Cite this article
Ebrahimi, S.M., Tebianian, M. Influenza A viruses: why focusing on M2e-based universal vaccines. Virus Genes 42, 1–8 (2011). https://doi.org/10.1007/s11262-010-0547-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-010-0547-7