Abstract
Based on the hypothesis that respiratory syncytial virus (RSV) sG protein causes allergy in patients, it is suggested that treatment of RSV patients with antagonists of IL-4 and FKN early in infection will prevent the increased level of IL-4 in the serum. Together with CpG ODNs that induce Toll-like receptor 9+ (TLR9+) plasmacytoid dendritic cells to release type I IFN-α and -β will reactivate the inhibited Th1 cells and the antiviral cytotoxic T leukocytes. In addition, binding of CpG ODNs to TLR9+ B cells will stop IgE synthesis and antiviral IgG and IgA will continue. Together, the IL-4 and FKN antagonists and CpG ODNs reactivate the adaptive immune response to clear the virus and protect the patient from a second RSV infection. It is also suggested that the less-pathogenic RSV strain Long may be a candidate for vaccine development after deletion of the FKN and superantigen domains from the G gene.
Similar content being viewed by others
Introduction
From the studies discussed in the respiratory syncytial virus (RSV) review [1] it is clear that a marked advance in the research on RSV has been made in the last 20 years. From the beginning of the clinical research on RSV-infected infants and children it was noted that a marked increase in IgE occurs in RSV patients, similar to the phenomenon in allergic inflammation. Since the mechanism by which allergens induce increased IgE levels was still unknown, clinicians assumed that allergic children are prone to RSV-induced bronchiolitis. In healthy children and adults B cells synthesize IgE at a low level, while in allergic individuals the level of IgE is markedly increased due to the response of the adaptive immune system to the presence of all sorts of allergens [2]. The allergens aggregate on IgE antibodies which are bound to the FcεRI+ hematopoietic cells (mast cells, basophils and monocytes) that respond by releasing large amounts of T helper 2 (Th2) cytokines IL-4, IL-5 and IL-13 from the preformed cytoplasmic granules, which skew the balance between Th1 ↔ Th2 cytokines toward Th2 > Th1, leading to the gradual inhibition of the humoral and cellular adaptive immune response.
The marked increase in the Th2 cytokine IL-4 causes B cells to stop IgG and IgA synthesis to switch to IgE synthesis. The increase of IgE level in RSV-infected individuals resembles the IgE increase in HIV-1/AIDS patients [3], and the findings that the viral inducer of allergy is the viral-shed gp120 molecules, as reported by Karray and Zouali [4], reviewed in reference [5]. Therefore, due to the resemblance in the responses of the innate and adaptive immune cells to HIV-1 and RSV, it is hypothesized that RSV codes for a glycoprotein that has allergen domain which binds to IgE VH3 sequence to release Th2 cytokines from FcεRI+ hematopoietic cells in addition to the Th2 cytokines produced by CD4+ Th2 cells. Kelly-Welch et al. [6] reported that IL-4 can stimulate two receptors, type I and type II, and IL-13 only type II. These cytokines activate the Janus kinase/signal transducer activator of transcription signaling cascades, which may contribute to allergic responses.
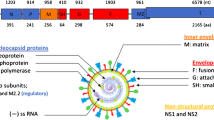
Three major structural glycoprotein genes are encoded by three RSV genes: (i) G glycoproteins, the full-length mG and truncated (from the N-terminus) soluble sG proteins; (ii) the fusion glycoprotein F and (iii) mSH glycoprotein. As reported above, the mG and the sG glycoprotein molecules contain two domains: the CX3C fractalkine motif and a T-cell antigen. To understand the properties of these two proteins computer programs were used to search for and identify a possible superantigen (allergen) domain.
RSV mG and sG glycoprotein molecules have the fractalkine and T-cell antigenic domains, the latter has the property of an allergen
The aa sequence of three RSV-A and three RSV-B isolates were compared using the computer program “Compare” (Fig. 1) to determine the identity and homology of the amino acids of both RSV strains. It was noted that three G proteins of RSV-A, 297, 298 and 298 aa in length, and RSV-B G proteins are 295, 295 and 299 aa in length (lacking four C-terminal aa) have high homology amongst the six aa sequences. In all RSV-A and -B strains G glycoprotein molecules the fractalkine motif is located between aa182CKSIC186 and the T-cell antigen domain is located in aa187KIIPSNKPKKKP198. In RSV-B G glycoprotein, the FKN domain is located at aa182CWAIC186 and the T-cell antigen is at aa187KRIPNKKPGKKT198.
The RSV mG gene codes for a full-length G glycoprotein and an N-terminus truncated sG glycoprotein. Computer analysis of full-length mG and sG molecules from RSV-A isolates were analyzed by multiple sequence alignment (Fig. 2). The first three sG glycoproteins are truncated at the N-terminus and are 124, 133, 133 aa in length. Two additional sG molecules are 251 and 250 aa in length. All the truncated RSV-A sG proteins conserved the CX3C FKN domain and the T-cell antigenic domain. This analysis suggests that the viral sG molecules are responsible for the induction of allergy and the eosinophilia in RSV-infected patients.
To determine if the T-cell antigen domain has an aa motif which can bind to the VH3 domain of IgE molecules, the aa sequence of the T-cell antigen in RSV-G was compared to the aa sequence of the superantigen domains in HIV-1 gp120 [4, 7, 8] (Table 1A, B). In these studies the authors designed synthetic peptides according to domains in HIV-1 gp120 and determined the amino acid motif that binds to IgE/FcεRI+, preventing induction of release of Th2 cytokines and histamine by gp120 molecules. Karray and Zouali [4] also used mutated peptides to identify the IgE VH3 binding domain in the gp120 peptides. Table 1A shows the gp120 peptide aa392–414 inhibited 70% of the cytokine and histamine release from mast cells by gp120. A mutated peptide in which the aa394 LFN 397 were replaced by three alanines, 394 AAA 397, inhibited the release of cytokines and histamine by 50%. The second domain of gp120 aa414–434, aa inhibited gp120 release of cytokines from mast cells by >70%. A mutant peptide in which aa425–427 were replaced by three alanines, aa425AAA427, lost its ability to block gp120 binding to IgE. These results indicated that three aa, LFN and IKQ, are the critical amino acids in the induction of allergy by HIV-1. Florio et al. [7] also identified domains in HIV-1 gp120 that were capable of interacting with IgE bound to basophils and causing cytokine release (not shown).
In Table 1B three gp120 peptides which were confirmed to contain superantigen domains were compared to RSV-G T-cell antigen domain. It can be seen that the RSV aa184–198 domain contains the aa sequence 125ICKRIP190 which resembles gp120 aa422PCRIK427 in peptides SP89261, SP89045 and 1960. The peptide RT33 of RSV [8] resembles the HIV-1 gp120 superantigen domain.
It is hypothesized that the RSV sG glycoprotein contains a superantigen motif in addition to the FKN motif which allows the sG proteins to bind to the VH3 domain of bound to FcεRI+ hematopoietic cells and cause the release of large amounts of Th2 cytokines to generate an allergy response. The increased IL-4 serum level inhibits the ability of Th1 cells to synthesize cytokines which activate pCTLs to become antiviral cytotoxic T leukocytes (CTLs). In addition, the sG glycoprotein FKN motif binds to the FKNRI expressed by the IL-5-activated eosinophils and direct their migration to the site of RSV infection and inflammation.
The molecular and immunological events during RSV primary infection in the respiratory epithelium leading to Th1 > Th2 cytokine response
The sequence of events that follow the binding of RSV virions to the respiratory ciliary epithelial cells of infants, children and the elderly during primary RSV infection is presented schematically in Fig. 3. RSV virions inhaled into the respiratory tract (Fig. 3a) bind to heparin on the respiratory ciliary epithelial cells and to the FKN receptors (FKNRI). Fusion of the viral and cellular envelopes, mediated by the F glycoprotein, enables the viral nucleocapsid to enter the cell cytoplasm, the site of viral RNA replication and viral protein synthesis. The first two genes that are expressed are NS1 and NS2, and inhibit the activation pathway of the cellular genes coding for the cellular type I IFNs. In the absence of the cellular IFN-α and -β, RSV replication and viral protein synthesis and virus assembly continue without delay. The infected cells which fuse to form syncytium release virions and the sG glycoprotein molecules. At this stage the innate and adaptive immune cells respond.
RSV primary infection of the respiratory epithelial cells leads to virus replication and the presentation of F peptides by HLA class I and sG glycoprotein molecules by HLA class II, leading to Th1/Th2 cytokine balance. a The respiratory ciliary epithelial cells fractalkine receptors bind virions. b DCs in the respiratory tract attract and mobilize RSV virions and sG proteins to the draining lymph nodes (LNs). c In the lymph nodes, DCs present viral antigen HLA class I and class II to polarize naïve T cells to become Th1 and Th2 cytokine producers (details in the text)
Figure 3b1 describes the response of the innate system dendritic cells (DCs) to the virus infection. The DCs in the respiratory tract compartment migrate to the site of RSV and with their mannose receptors, DC-SIGN, bind RSV virions and the virus F and sG glycoproteins. The DCs phagocytise virions, viral proteins and infected cell debris, internalize their dendrites and become rounded cells with a “sail” (designated “veiled” cells). These cells access the lymph vessels and migrate with the lymph current during a 24-h period in the direction of the draining lymph nodes. During this time viral proteins are degraded by the cytoplasmic proteasomes, and HLA class I peptides are directed to the endoplasmic reticulum to bind to the assembling HLA class I molecules. The HLA HLA molecules are transferred to the DC dendrites, allowing binding of the naïve T0 cells. The bone marrow-derived innate system cells—mast cells, basophils and monocytes, migrate to the vicinity of blood vessels. These cells are FcεRI+ cells and contain granules filled with Th2 cytokines, histamine and prostaglandin. These cells will be induced to release the cytokines by allergens which aggregate with IgE bound to FcεRI+ hematopoietic cells [Fig. 3b2].
Figure 3c1 describes the events occurring after the arrival of the virion-loaded DCs into the T-cell compartment of the lymph node. The DCs spread their dendrites, presenting the peptide-loaded HLA class I, class II and CD1 molecules. The latter present lipids.
The CD4+ T cells in the lymph node are naïve T cells which have arrived from the thymus after interaction with the thymus stromal epithelial cells which express the entire human proteome that guides T-cell development and selection, enabling them to identify foreign antigens [9]. The naïve Th0 cells that bind to the DC HLA class I molecules are polarized within 24 h into Th1 cells and the synthesis and release of the Th1 proinflammatory cytokines IL-2, IL-12 and IFN-γ is activated. The naïve T cells that bind to HLA class II are polarized to become Th2 cells which produce IL-4, IL-5, IL-10 and IL-13, the Th2 inflammatory cytokines. The CD4+ Th1 cells and CD4+ Th2 cells express the IL-4 receptor (IL-4Rα). The Th1 cytokines activate the CD8+ T-cell precursors to become CTLs, while the inflammatory cytokines IL-4 and IL-10 inhibit the synthesis of the proinflammatory cytokines when the Th1 ↔ Th2 cytokine balance is skewed toward Th > Th1 imbalance.
It is suggested [Fig. 3c1] that during primary RSV infection the Th2 cytokine level is lower than the Th1 cytokine level (Th1 > Th2) and the IL-4 cytokine released by CD4 T cells does not prevent the Th1 cell induction of antiviral CTLs and does not induce B cells [Fig. 3c2] to synthesize anti-RSV neutralizing IgG and IgA antibodies and memory T cells. The increased Th1 level during a primary RSV infection in infants, children and the elderly leads to activation of the adaptive immunity and recovery.
The impact of RSV-G, sG and F glycoproteins on the innate and adaptive immune response during a secondary RSV infection
Respiratory syncytial virus infection leads to bronchiolitis and wheezing. RSV infection of the ciliary epithelial cells in the respiratory tract may lead to increased release of sG glycoprotein from the infected cells that DCs process and present by HLA class II molecules to a large number of naïve T cells in the lymph nodes. These polarized cells produce an increased amount of IL-4 by CD4+ T cells by inducing the synthesis of the IL-4-responsive genes, IL-4Rα, IL-4, Iε (precursor of IgE, HLA class II and FcεRI) [Fig. 4b1]. The increased IL-4 level affects Th1 cells that were polarized by DCs presenting RSV-F peptides by binding to their IL-4Rα, inhibiting their ability to synthesize and release Th1 cytokine. As a result, the patient’s cellular immune response is gradually inhibited [Fig. 4b2]. At the same time, the gradual increase of IL-4 affects the ability of B cells to synthesize IgG and IgE, since IL-4 binding to IL-4Rα on B cells causes cessation of IgG and IgA synthesis and the B cells switch to IgE synthesis (Fig. 4c). The gradual increase in IgE in the serum gradually engages FcεRI+ hematopoietic cells by binding to the IgE receptor. RSV sG glycoproteins bind with its superantigen domain to the VH3 domain of IgE/FcεRI+ hematopoietic cells. The cells release large amounts of IL-4 which leads to complete shut-off of the patient’s adaptive immunity and leads to immune deficiency (Fig. 4a).
The increased levels of IL-5 activate the innate system IL-5R+, FKNRI+ and eosinophils which migrate to the inflamed respiratory tract and cause bronchiolitis and wheezing (Fig. 4d). Antiviral IgE antibodies are not neutralizing and serve as an indicator of the virus-induced allergy (Fig. 4d). In addition to the marked increase of Th2 cytokine induction by sG glycoprotein, IgE/FcεRI+ hematopoietic cells release large amounts of histamine and prostaglandin F2 alpha metabolite (PGm) by mast cells and basophils that have a role in the induction of acute bronchiolitis and wheezing in infants and children [10].
A novel approach to prevent RSV-induced allergy by combined treatment with IL-4 antagonist IL-4δ2, fractalkine antagonist together with CpG ODN
The damage to the cellular and humoral adaptive immune system is caused by RSV expressing the NS1/NS2 genes as the first genes in the infected cells to prevent the cells from inducing type I IFN genes. By doing so, the infected respiratory epithelial cells are unable to resist the virus infection or alert neighboring cells to develop resistance to infection. The second tool that RSV utilizes is the presence of two initiation sites for transcription of the G glycoprotein gene to generate a full-length G glycoprotein and truncated sG glycoproteins that are released early in infection from the infected cells. These sG molecules have a fractalkine CX3C chemokine motif and a T-cell antigen domain which mimics an allergen. The employment of NS1/NS2 genes allows the virus to replicate and release the sG molecules to subdue the patient’s adaptive immunity.
It is suggested that in order to prevent RSV infection it is necessary to focus our attention on preventing RSV from infecting the ciliary respiratory cells, to prevent the polarization of Th2 cells and the increase in the Th2 cytokine level, especially that of IL-4, and to prevent the ability of sG molecules to bind to IL-5R+ FKNRI+ eosinophils. It is possible to achieve the goal by the use of cytokine and chemokine antagonists that will block the critical receptors on the different cell types, and together with CpG ODN, cause the innate system plasmacytoid DCs to (PDC) release large amounts of type I IFN that will reactivate the inhibited adaptive immune response of the patient.
The splice variant of IL-4, IL-4 delta 2, binds to IL-4R alpha on CD4+ Th2, CD4+ Th1 cells and B cells and prevents the skewing of the Th2 cytokine level
The human IL-4 gene (hIL-4), a single copy gene in the haploid genome, was mapped to chromosome 5. Arai et al. [11] cloned and sequenced the gene and reported that it contains four exons and three introns and is approximately 10 kb. The X-ray crystal of IL-4 at 235 Å was reported by Walter et al. [12] to be four alpha-helices (58% of the structure). The helices are arranged in a left-handed anti-parallel bundle with two overhead connections. Within these connections is a two-stranded anti-parallel beta-sheet. IL-4 binds to IL-4R alpha with the surface residues of helix A, loop AB and the C-terminal end of helix C and D. Alms et al. [13] reported that T cells stimulated by OKT3 monoclonal antibodies expressed IL-4 and the splice variant IL-4δ2. The expression of IL-4 and IL-4δ2 in healthy individuals is in the range of 16:1 to 1:1, while three individuals expressed more IL-4δ2 mRNA than IL-4 mRNA. Klein et al. [14] reported that IL-4 and IL-4δ2 were expressed in all leukemic and lymphoma cell lines after PML stimulation. Arinobu et al. [15] reported that rhIL-4δ2 blocked rhIL-4 activity by binding to IL-4Rα on monocytes (450 receptors per cell) and prevented rhIL-4 binding to IL-4Rα. The effect of IL-4δ2 on IgE synthesis by B cells after treatment with IL-4 was also studied. The synthesis of IgE was found to be reduced by the addition of the IL-4 antagonist in a dose-dependent manner.
Individuals capable of producing more IL-4δ2 than IL-4 were reported to control Mycobacterium tuberculosis (TB) [16], while Fletcher et al. [17] reported that PBMCs from healthy TB contacts release IFN-γ in response to M. tuberculosis-specific secreted ESAT-6 antigen also produce higher levels of IL-4δ2 mRNA than ESAT-6-negative donors. Seah et al. [18] studied PBMCs from patients with atopic asthma, TB-infected and healthy individuals. The authors reported that IL-4δ2 expression in cells from asthmatic patients was 2.8 logs higher than from cells of TB patients, and 4.5 logs higher than healthy individuals. The IL-4δ2 in individuals which control TB infections is higher than in TB-diseased individuals.
It is proposed that treatment of infants and children with IL-4δ2 prior to RSV or early in RSV infection may reduce the severity of the disease by the mechanism described in Fig. 5 (1) (Treatment with IL-4δ2). All cells in the virus-infected respiratory tract which express IL-4Rα will bind the antagonist and will prevent the allergic response caused by the viral sG glycoprotein.
In addition to the natural IL-4 delta 2, Avensa et al. [19] engineered a mutation, Tyr(124)Asp, in synthetic IL-4 molecules and reported that the synthetic antagonist prevented IL-4 induction of IgE synthesis in human B cells. This study indicates that synthetic IL-4 antagonists could be developed.
CX3C chemokine fractalkine antagonists will prevent RSV virion attachment by the mG glycoprotein to FKNRI+ respiratory epithelial cells and will prevent the trafficking of IL-5-activated eosinophils to the inflamed respiratory tract
Treatment with FKN antagonist binds to FKNRI+ on ciliary respiratory epithelial cells and will prevent RSV attachment to its specific cellular receptor and reduce the number of infected cells in the respiratory tract [Fig. 5 (2)].
Development of FKNR1 inhibitory molecules requires information on the solution structure of the CX3C chemokine domain of FKN and its interactions with an N-terminus of CX3CR1. Mizoue et al. [20] studied the solution structure of the chemokine domain of FKN, aa residues 1–76, by the heteronuclear NMR method. The authors compared CX3C FKN structure to that of CC and CXC chemokines and found differences relevant to receptor binding: there is a bulge formed by the CX3C motif, the relative orientation of the N-terminus and 30’s loop (aa30–38) and the conformation of the N-loop (aa9–19), regions of the protein that are dynamic. The FKNR1 contacts the fractalkine CX3C domain that maps roughly to the regions of greatest flexibility and structural variability.
Inoue et al. [21] developed FKN analogs by truncating ≥4 aa from the N-terminus and reported that the truncated FKN failed to induce chemotaxis and calcium influx by CX3CR1-expressing cells. The most potent antagonist (FKN-AT) lacked the four N-terminal aa. The authors used this FKN-AT to inhibit FKN expression in the glomerular endothelial cells of 12-week-old MRL/lpr mice, suffering from the autoimmune disease systemic lupus erythematosus. MRL/lpr mice spontaneously develop lethal glomerular diseases with an increase in circulating immune complexes, autoantigen production and cytokine abnormalities. FKN is expressed at a very low level by resting epithelial cells, but undergoes marked stimulation by cytokines like TNF-α and IL-1β. The authors reported that FKN-AT delayed the initiation and ameliorated the progression of murine lupus nephritis.
Hasegawa et al. [22] used MRL/lpr mice at 7 and 12 weeks of age and injected MRL/N-1 cells that were transfected with plasmids expressing NH2-terminal truncated monocyte chemoattractant protein 1 (MCP-1/CCL2) or thymus and activation-regulated chemokine (TARC/CCL17) analogs. The authors reported that after 8 weeks, mice bearing MCPA antagonist showed markedly diminished infiltration of macrophages and T cells, glomerular hypercellularity and vasculitis compared to control mice due to decreased production of IFN-γ and IL-2. There was no significant difference in renal damage between mice treated with TARC antagonist and control mice.
Davis et al. [23] reported that the human herpesvirus-8 (latent in Kaposi’s sarcoma cells)-encoded chemokine is the viral-coded macrophage inflammatory protein (vMIP)-II. This viral protein is a non-selective chemokine receptor antagonist including CX3C FKNRI due to its structural similarity to FKN. The authors aimed to change the vMIP-II FKN domain to make the antagonist a specific inhibitor of FKNRI. Chimeric and insertional mutagenesis was used to generate mutants of both vMIP-II and FKN. The expressed proteins were evaluated for chemokine receptor binding affinities and CX3CR1 binding efficacy. The modification of the amino acids between the first conserved cysteine residues of FKN and vMIP-II revealed the role of the X3 bulge of FKN in the affinity to CX3CRI. Substitution of vMIP-II N-terminus with that of FKN created an agonist that was as effective as FKN in binding CX3CRI. However, replacement of the FKN N-terminus with the same domain of vMIP-II disrupted the ability of chimeric FKN to bind the FKN receptor. The authors concluded that “the development of specific chemokine receptor antagonists based upon virally encoded chemokine peptides offers the potential for improved strategies for targeting inflammatory mechanisms associated with human disease.” This approach is based on the finding of Crump et al. [24] who studied the structure and function of human herpesvirus-8 MIP-II (aa1–71) and the N-terminal segment (aa1–10), a broad range chemokine antagonist. The authors studied two N-terminal peptides, V-MIP-II (aa1–10) and vMIP (aa1–11) dimer (dimerized through cysteine 11). Both peptides bind to CXC chemokine receptor 4 (CXCR4, the HIV-1 receptor expressed by CD4+ T cells). In contrast, vMIP-II (aa1–10) was 1.4×103-fold less potent than the native protein, while vMIP-II (aa1–11) dimmer was only 180-fold less potent. It was reported that the N-terminus of vMIP-II N-terminus over aa5–8 is a turn-like structure. This feature was not observed during the application of standard NMR methods for solving the full protein structure and requires two-dimensional methods.
Treatment of RSV-infected patients with CpG ODN to reactivate the RSV-inhibited cellular and humoral immune responses
The use of IL-4 delta 2 and FKN antagonists are important due to their ability to prevent IL-4-induced allergy and eosinophilia in RSV patients. The addition of CpG ODN to treatment with the two antagonists is needed for induction of type I IFN-α/β release from PDCs that will activate the IL-4-inhibited Th1 cells to release cytokines and IL-4-inhibited B cells to reactivate the antiviral IgG synthesis and inhibition of IgE synthesis. Figure 5 (3) (treatment with CpG ODN) shows the sequence of the events leading to the reactivation of the adaptive immune response.
CpG ODNs, non-methylated bacterial-like synthetic DNA, bind to Toll-like receptor 9+ (TLR9+) PDC which respond by releasing large amounts of preformed type I IFN-α/β. The IFNs induce the IL-4-inhibited Th1 cells to reactivate and produce Th1 cytokines to resume the activation of precursor CTLs to become anti-RSV CTLs. CpG ODNs also bind to TLR9+ B cells and inhibit IgE synthesis and the release of Th2 cytokines from FcεRI+ hematopoietic cells. CpG ODNs also inhibit viral RNA replication. Experimental studies provided information on the ability of CpG ODNs to treat allergy in animals.
Tayyari et al. [25] evaluated the immunotherapy capability of CpG ODN, a potent Th1 stimulant, on ovalbumin sensitization of guinea pigs with and without RSV infection. The authors measured the histology of the lungs for airway inflammation and the Th1/Th2 balance (IFN-γ/IL-5 mRNA ratios), ovalbumin (OA)-specific IgG antibodies. RSV antigens in the infected lung tissue sections were identified by immunohistochemistry. CpG ODN immunotherapy did not prevent OA sensitization of the animals. However, in RSV-infected, treatment of OA-sensitized animals treatment with CpG ODN caused significant reduction of airway T cells and eosinophils, increased lung IFN-γ/IL-5 ratio and decreased OA-specific IgG1 antibodies to the levels in uninfected, OA-sensitized animals. Viral antigens were identified in the lungs. The authors concluded that CpG ODN treatment protected guinea pigs against RSV infection.
Schlender et al. [26] studied the inhibition of TLR7- and TLR9-mediated type I IFN production by PDCs by RSV and the measles virus, which cause Th2-biased immune response in infected people. The authors studied RSV strain “Long” and reported that this virus strain is an efficient inducer of type I IFN release by PDC and reported that infection of PDCs with RSV strain A2 or measles virus “Schwarz” strain inhibited the induction of IFN-α and -β induced by treatment with CpG ODN. The TLR9 agonist CpG ODN-A and the TLR7 agonist, resiquimod (R848), induced PDCs to release large amounts of type I IFN-α, β. Mock-infected PDCs, after a 6-h CpG ODN incubation period, secreted 2, 4 and 13 ng/ml IFN-α at 12, 24 and 36 h, respectively. Infection with RSV “Long” caused slightly increased levels of IFN-α, and RSV-A2 and measles virus strongly diminished IFN-α secretion from PDCs treated for 6 h with CpG ODN. Compared to mock- and RSV “Long”-infected PDCs, a reduction of IFN-α levels to less that 50% was observed with RSV-A2-infected PDCs suggesting that RSV “Long” NS1 or NS2 activity differed from RSV-A2.
These studies suggest that treatment of RSV-infected animals with CpG ODN induced the PDCs to release IFN-α and -β in the respiratory epithelium at a level of 13 ng/ml 24 h after treatment which sufficed to inhibit RSV replication. A low level of IFN-α, β most probably does not induce autoantibodies. Our hypothesis suggests that a combined treatment with IL-4 and FKN antagonists, together with CpG ODN-C, may completely prevent and inhibit RSV replication in the ciliary epithelial cells and the skewing of the Th1/Th2 balance toward Th2 cytokines. IFN-α, β release from CpG ODN-treated PDCs will activate Th1 cells affected by the increase in IL-4 to synthesize and release the Th1 cytokines IL-2, IL-12 and IFN-γ that will reactivate the anti-RSV cellular response. CpG ODN binding to the TLR9+ B cells will inhibit IgE synthesis and induce the synthesis of antiviral humoral antiviral IgG and IgA antibodies. The combined treatments with IL-4l2, fractalkine antagonists and type I IFN inducer will reactivate the patient’s adaptive immunity to clear the virus infection.
Conclusions and implications
Advances in the research on RSV have provided information on the viral genes and proteins and their role in evading the infected individual’s adaptive immune response. It has been shown that the NS1/NS2 genes, the first to be transcribed from the viral genes, are responsible for the virus-induced inhibition of the cellular genes coding for the cellular type I IFNs. Thus, immediately after infection the infected ciliary respiratory cells are unable to stop RSV replication and cannot signal neighboring uninfected cells to resist the virus infection. Subsequently, in the absence of the cellular IFNs, the rest of the viral genes are expressed and viral proteins are synthesized, leading to the release of infectious enveloped virions which contain the viral glycoproteins G, F and SH. The G (attachment) glycoprotein gene has two initiation sites for translation which produce the full-length G glycoprotein (mG) and the N-truncated soluble G (sG). Both mG and sG glycoproteins contain two domains: the CX3C fractalkine (FKN) domain and the T-cell antigen, which has a domain of an allergen. The FKN domain of sG is responsible for directing IL-5-activated eosinophils to migrate to the site of inflammation cause by RSV infection. More important is the T-cell antigen domain which resembles the allergen sequence that binds to the IgE VH3 domain attached to FcεRI hematopoietic cells. With the sG allergen sequence RSV skews the Th1 ↔ Th2 balance toward Th2, causing allergy in the patients which inhibits the adaptive immune response.
Based on the understanding that increased Th2 cytokine IL-4 is responsible for the inhibition of the cellular and humoral responses of the adaptive immune system of infants, children and the elderly, it is suggested that development of treatments with IL-4δ2 antagonist, together with FKN antagonist and CpG ODN-C that will inhibit the RSV-induced allergy and will reactivate the patient’s adaptive immune response to clear the virus infection, may ameliorate the infection. Further studies on cytokine and chemokine antagonists and CpG ODN-C are necessary.
The identification of the viral F protein involvement in the induction of Th1 cytokine synthesis suggested that this glycoprotein is beneficial for the infected patients, while the fact that the sG glycoprotein is detrimental implies that for the development of a pathogenic RSV vaccine strain, “Long” for example, the G protein domains FKN and T-cell antigen (superantigen), as well as the second initiation codon for sG, should be deleted. The deletion of NS1/NS2 genes has already been suggested, but the complete deletion of these genes allows cellular type I IFN synthesis, resulting in inhibition of RSV replication. It is suggested that partial deletion of the NS1/NS2 domains which are involved in IFN pathway inhibition should be deleted.
References
Y. Becker, Virus Genes (2006, in press)
O. Mirza, A. Henriksen, H. Ipsen, J.N. Larsen, M. Wissenbach, M.D. Spangfort, M. Gajhede, J. Immunol. 165, 331–338 (2000)
J. Wilson, K. Rowlands, K. Rockett, C. Moore, E. Lockhart, M. Sharland, D. Kwiatkowski, J. Hull, J. Infect. Dis. 191, 1705–1709 (2005)
S. Karray, M. Zouali, Proc. Natl. Acad. Sci. U.S.A. 94, 1356–1360 (1997)
Y. Becker, Virus Genes 28, 319–331 (2005)
A.E. Kelly-Welch, E.M. Hanson, M.R. Boothby, A.D. Keegan, Science 300, 1527–1528 (2003)
G. Florio, A. Petraroli, V. Patella, M. Triggiani, G. Marone, AIDS 14, 931–938 (2000)
R.A. Tripp, L.P. Jones, L.M. Haynes, H.-Q. Zheng, P.M. Murphy, L.J. Anderson, Nat. Immunol. 2, 732–738 (2001)
Y. Takahama, Nat. Rev. Immunol. 6, 127–135 (2006)
D.P. Skoner, P. Fireman, L. Caliguiri, H. Davis, Am. Rev. Respir. Dis. 142, 359–364 (1990)
N. Arai, D. Nomura, D. Villaret, R. DeWaall Malefijt, M. Seiki, M. Yoshida, S. Minoshima, R. Fukuyama, M. Maekawa, J. Kudoh, N. Shimizu, K. Yokota, E. Abe, T. Yokota, Y. Takebe, K. Arai, J. Immunol. 142, 274–282 (1989)
M.R. Walter, W.J. Cook, B.J. Zhao, R.P. Cameron Jr., S.E. Ealick, R.L. Walter, P. Reichert, T.L. Nagabhushan, P.P. Trotta, C.E. Bugg, J. Biol. Chem. 267, 20371–20376 (1992)
W.J. Alms, S.P. Atamas, V.V. Yurovsky, B. White, Mol. Immunol. 33, 361–370 (1996)
S.C. Klein, J.G. Golverdingen, D.F. van Wichen, A.G. Bouwens, I. Stuij, M.G.J. Tilanus, E.J. Bast, R.A. de Weger, Cell. Immunol. 167, 259–268 (1996)
Y. Arinobu, S.P. Atamas, T. Otsuka, H. Niiro, K. Yamaoka, H. Mitsuyasu, Y. Niho, N. Hamaski, B. White, K. Izuhara, Cell. Immunol. 191, 161–167 (1999)
A. Demissie, M. Abebe, A. Aseffa, G. Rook, H. Fletcher, A. Zumla, K. Weldingh, I. Brock, P. Andersen, T.M. Doherty, VACSEL Study Group, J. Immunol. 172, 6938–6943 (2004)
H.A. Fletcher, P. Owiafe, D. Jeffries, P. Hill, G.A. Rook, A. Zumla, T.M. Doherty, R.H. Brookes, The VACSEL Study Group, Immunology 112, 669–673 (2004)
G.T. Seah, P.S. Gao, J.M. Hopkin, G.A.M. Rook, Am. J. Respir. Crit. Care Med. 164, 1016–1018 (2001)
G. Avensa, J. Punnonen, B.G. Cocks, R.W. de Waal Malefyt, F. Vega Jr., S.M. Zurawski, J.E. de Vries, J. Exp. Med. 178, 2213–2218 (1993)
L.S. Mizoue, J.F. Bazan, E.C. Johnson, T.M. Handel, Biochemistry 38, 1402–1414 (1999)
A. Inoue, H. Hasegawa, M. Kohno, M.R. Ito, M. Terada, T. Imai, O. Yoshie, M. Nose, S. Fujita, Arthritis Rheum. 52, 1522–1533 (2005)
H. Hasegawa, M. Kohno, M. Sasaki, A. Inoue, M.R. Ito, M. Terada, K. Hieshima, H. Maruyama, J.I. Miyazaki, O. Yoshie, M. Nose, S. Fujita, Arthritis Rheum. 48, 2555–2566 (2003)
C.N. Davis, V. Zujovic, J.K. Harrison, Mol. Pharmacol. 66, 1431–1439 (2004)
M.P. Crump, E. Elisseeva, J.H. Gong, I. Clark-Lewis, B.D. Sykes, FEBS Lett. 489, 171–175 (2001)
F. Tayyari, T.C. Sutton, H.E. Manson, R.G. Hegele, Eur. Respir. J. 25, 295–302 (2005)
J. Schlender, V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, K.K. Conzelmann, J. Virol. 79, 5507–5515 (2005)
Acknowledgments
The author is indebted to Professor G. Darai, Hygiene Institute Karl-Ruprecht University in Heidelberg, Germany, for his suggestions and advice, and to Aviad Levine, M.Sc. for his help with the computer analyses and the graphic design of the figures and table.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Becker, Y. Respiratory syncytial virus(RSV)-induced allergy may be controlled by IL-4 and CX3C fractalkine antagonists and CpG ODN as adjuvant: hypothesis and implications for treatment. Virus Genes 33, 253–264 (2006). https://doi.org/10.1007/s11262-006-0063-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-006-0063-y