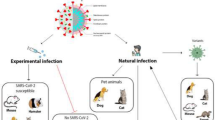
Abstract
The outbreak of the SARS-CoV-2 in mainland China with subsequent human to human transmission worldwide had taken up the shape of a devastating pandemic. The ability of the virus to infect multiple species other than humans has currently been reported in experimental conditions. Non-human primates, felines, ferrets, rodents and host of other animals could previously be infected in experimental conditions with SARS-CoV and recently with SARS-CoV-2, both virus using Angiotensin-converting-enzyme 2 receptor for cellular entry. The variations in sequence homology of ACE2 receptor across species is identified as one of the factors determining virulence and pathogenicity in animals. The infection in experimental animals with SARS-CoV or SARS-CoV-2 on most occasions are asymptomatic, however, the virus could multiply within the respiratory tract and extra-pulmonary organs in most of the species. Here, we discuss about the pathogenicity, transmission, variations in angiotensin-converting-enzyme 2 receptor-binding across species and host pathogen interactions of SARS and SARS-CoV-2 in laboratory animals used in research.
Similar content being viewed by others
Introduction
Coronaviruses (CoVs) are a family of viruses which were known to cause mild flu like symptoms in humans previously. However, these viruses harboured in wild mammals when transmitted to humans resulted in two pandemics of severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS) during the last two decades. The outbreak of severe acute respiratory syndrome corona virus 2 (SARS-CoV-2), popularly known as coronavirus disease 19 (COVID19) is the reoccurrence of infection by SARS related viruses which has been declared by the World Health Organisation (WHO) as a global pandemic. The outbreak occurred in a local sea food market in Wuhan, Hubei province in central China from a probable bat origin (Zhou 2020) which had a human to human transmission to more than 150 countries resulting in severe morbidity and mortality. The check of human to human transmission is only possible at present by following a set of hygienic principles, social distancing, isolation and quarantine of suspected patients as there are no validated therapeutics or a vaccine for prevention of the disease. Inoculation of SARS-CoV-2 in experimental animal reveal susceptibility of infection in cats, ferrets, dogs, hamsters, mouse and NHPs. The research revealing infectivity, pathogenicity and virulence of emerging infectious disease of SARS-CoV and SARS-CoV-2 on experimental animals for development of drugs, identification of therapeutic targets, vaccine trials etc. are gaining momentum. Here, we review some similarity and differences with animal models of SARS-CoV and SARS-CoV-2 infections as both the virus shares a homogeneity in infection pattern, pathogenicity, transmission and ACE2 receptor binding domains of spike protein.
Threats arising out of SARS-L-CoV outbreaks
Coronaviruses capable of infecting humans have been reported as early as 1890s causing flu like symptoms. Prior to outbreak of COVID-19, six CoV strains affecting humans have been reported most of which are harbored in bats and some in rodents (Cui 2019) (Table 1). The outbreak of SARS in 2002 exhibited the ability of CoVs to cause widespread pandemic in human population, however the outbreaks were contained within a span of two years. Initially the palm civets were thought to be the reservoir host of this deadly virus, however when civets were inoculated with field isolates of human SARS-CoV rendered them infected indicating that civets are not the natural host of these CoVs (Wu 2005). Sample survey of 408 bats in 2004 from multiple species, genera and families in different provinces of China indicated a widespread seropositivity of SARS-CoV infection in bats indicating that bats are the reservoir host of several CoVs having the ability to cause severe respiratory syndromes (Li 2005b, Lau 2005). Nasopharyngeal, anal swabs and blood collected from 127 bats, 60 rodents and 20 monkeys at HKSAR region between 2004 and 2005 and subsequent isolation of CoVs revealed that 39% of Chinese horse shoe bats possess CoVs similar to SARS-CoVs. The genome analysis of the CoVs reveled the striking similarity of bat SARS-CoVs with that of human and civet SARS-CoVs (Lau 2005). Whole genome sequence analysis of two novel CoVs (RsSHC014 and Rs3367) isolated from Chinese horse shoe bats of Rhinolophidae family were closely related to the SARS-CoV specially with respect to the receptor binding domain (RBD) of the viral spike protein, and that CoVs of Chinese horse shoe bats can directly be transmitted to humans without any intermediate host was recognized (Ge 2013). Identification and characterization of CoVs circulating in Chinese horse shoe bats possessed a severe threat to human population as evident from data and metagenomics study results obtained during the past 5 years (Ge 2013, Menachery 2015, He 2014).
SARS coronaviral infections and pathogenicity in experimental animals
The threats from CoVs had urged the scientific community to search for therapeutics, vaccines and diagnostics from the very initial stage resulting in experimental infection of animals with human or field isolates of the virus. The novelty of CoVs and differences in receptor binding domains of viral spike protein across multiple species was elusive on several occasions. SARS-CoVs uses the ACE2 receptor for entry into cells. Sequence homology of both ACE2 receptor varies across species and hence exhibition of human like symptoms in animals is not always observable in case of infection. Non-human primates (NHP) like African green monkeys (AGM), rhesus monkeys and cynomolgus monkeys when inoculated with of SARS-CoVs failed to show clinical signs of infection in all the species (only 1 out of 4 AGM showed high rectal temperature). Viral replication was evident in all the three species of monkeys and the replication was found to be higher in AGMs as compared to rhesus and cynomolgus. The early infection of broncho-tracheal epithelial cells and type 1 pneumocytes were also evident and consistent with observations in human SARS-CoV infection (McAuliffe 2004). Common marmosets infected with SARS-CoV showed mild clinical signs and symptoms of respiratory illness. Non-hemorrhagic diarrhea was observed in 7 out of 12 infected animals. Viral antigens could be detected in alveolar cells and type 1 pneumocytes along with multiple tissues indicating human like tissue distribution and viral multiplication of SARS-CoV (Greenough 2005). Cats and ferrets when inoculated with SARS-CoV did not show any clinical symptoms of illness although viral RNA could be detected in lungs, tracheobronchial lymph nodes and heart depicting asymptomatic illness (van den Brand 2008). Pigs and chickens were also asymptomatic after inoculation with human isolates although viral RNA could be detected in spleen in pigs and in blood, respiratory tract, kidneys of chicken (Weingartl 2004). Mice infected with SARS-CoV showed clinical signs of illness replicable of human like symptoms but young mice but do not show any clinical signs of illness, although virus multiplies in their respiratory tract (Roberts 2005a; Subbarao 2004). In a recent study, mice inoculated with an emerging strain of CoV WIV1-MA15, a bat origin novel CoV showed no symptoms or lethality of infection (Menachery 2016). Golden hamsters inoculated with a SARS-CoV strain shows no clinical signs of disease although viral RNA could be detected in respiratory tract (Roberts 2005b). The susceptibility, replication and pathogenicity is detailed in Table 2.
SARS-CoV-2 infection and pathogenicity in experimental animals: recent findings
Recent studies indicate that ferrets are susceptible to SARS-CoV-2 infection with viral isolates from environmental and patient samples. The virus could be replicated in the upper respiratory tract and the virulence was restricted to mild disease without death. SARS-CoV-2 could bind to epithelial cells and type II pneumocytes of the lungs in ferrets but could not replicate within the lungs indicating that infection was restricted only to upper respiratory tract in ferrets (Shi 2020). Identical studies conducted in sub adult cats revealed that droplet transmission of SARS-CoV-2 can occur in cats and they are susceptible for infection however, infection is limited to upper respiratory tract in cats. Interestingly juvenile cats when inoculated with SARS-CoV-2 resulted in death or severe morbidity and histopathological lesions were observed in both upper and lower respiratory tract (Shi 2020). Dogs are mildly susceptible to SARS-CoV-2 infection whereas similar studies conducted on pigs, ducks and chickens revealed no susceptibility of these species to SARS-CoV-2 infection (Shi 2020). Syrian hamsters when inoculated with SARS-CoV-2 showed similarity in disease pathogenesis, progression and transmissibility as observed in human infections. Severe infection of both upper and lower respiratory tracts, histopathological findings, cytokine and chemokine levels and changes in extra pulmonary organs were also evident (Chan 2020). Rhesus monkeys infected with SARS-CoV-2 showed viral presence in respiratory tract and gut (Bao 2020a). The findings are very necessary and important in the race for developing a therapeutic drug or vaccines against SARS-CoV-2. The susceptibility, replication and pathogenicity is detailed in Table 3.
Host pathogen interactions of SARS-CoVs in experimental animals with respect to ACE2 RBD
SARS-CoVs are assumed to have been originated through natural sources from other mammalian species (most likely from bat), it failed to mimic human clinical disease in animals and thereby limiting in depth of knowledge on infection process and possible modes of prevention and cure of this infection. The virus only exhibited transient viremia and milder symptoms in many of the model species while in some cases experimental infection ended without any clinical symptom or histopathological changes. In this context, high affinity virus receptor interaction might be one of the crucial factors that determines virulence of the pathogen in the host. This can be justified by the fact that experimental infection of hACE2 (functional receptor of SARS-CoV) expressing transgenic mice show clinical manifestations similar to human infection in comparison to the parental wild type mice (Tseng 2007). Likewise, murine 3T3 cells expressing human ACE2 were more susceptible to SARS-CoV infection as compared to murine ACE2 (Li 2004). Host cellular invasion of SARS-CoV is mediated through interactions among distinct receptor-binding domains (RBD) on the SARS-CoV spike (S) protein and its functional receptor ACE2 wherein S protein binding domains are localized at 30–41 amino acid (aa) and 82–84 amino acid of ACE2 (Li 2005a). Therefore, the likelihood that sequence homology at S protein binding domains is the key determinant on susceptibility and clinical manifestation of SARS-CoV in animals. In line with this hypothesis, analysis of human ACE2 protein sequence with other potential host (experimental and reservoir) animals revealed more homology in bat vs. human and rhesus monkey than bat vs. other mammals at 30–41 aa region (Fig. 1). However, human and monkey ACE2 showed relatively less homology towards bat ACE2 at 82–84 aa region than cat, dog, ferret, marmoset, civet and raccoon. In both the S protein binding regions of ACE2, mouse and rat displayed distant homology for bat ACE2 than other species. Thus, it is likely that higher homology at binding domains in ACE2 receptor enabled SARS-CoVs for cross species infection from reservoir host to human during close proximity of human and bat in food markets in China. Similarly, the distant homology at S protein binding domains in mouse and rat ACE2 with bat and human ACE receptor possibly explains limited success in developing representative animal models for in depth study of SARS-CoV-2 infection. However, other host factors such as age, virome, host genetics, immune response pattern, previous and concomitant infections etc. may also play important role in mild or lack of clinical symptoms in animals (Sumbria 2019). Likewise, influence of other receptors like CD209L (Jeffers 2004) and Aminopeptidase N (APN) (Wentworth 2001) in efficiency of infection and replication of SARS-CoV in other host species cannot be excluded.
Alignment of ACE2 amino acid sequences of different mammals. Analysis was performed by Clustal Omega programme using SnapGene software. Gene bank accession number of ACE2 proteins for indicated species are- H. sapiens, AB193260; R. leschenaultia, AB299376; M. mulatta, FJ170076; C. jacchus, XM_008988993.1; P. larvata, AY881174; N. procyonoides, EU024940; Mustela putorius furo, AB208708; F. catus, AB211997.1; Canis lupus familiaris, NM_001165260; R. norvegicus, AY881244; M. musculus, AK141604; B. taurus, NM_001024502.4.; B. bubalis, XM_006041540.2; C. hircus, XM_005701072; O. aries, XP_011961657
One of the conventional way of developing animal models of viral infection is adaptation of the pathogen in laboratory animal by serial passaging. This will eventually enable the virus to gain virulence in the animal host and thereby represent physiological responses of regular infection. Serial passaging of wild type virus in unnatural host also reduces its virulence towards normal host and is the traditional method of development of live attenuated vaccine for original host. However, adaptation of human virus in different host species may also impose potential risk of evolution of new viral strain which in turn may cause new epidemic.
hACE2 transgenic mice in SARS-CoV and SARS-CoV-2 experimentation
The SARS-CoV and SARS-CoV-2 uses the ACE2 receptor for cellular entry hence transgenic mice bearing hACE2 receptor is reported to be a good model for SARS-CoV multiplication. K18-hACE2 mice when inoculated with SARS-CoV isolates showed signs and symptoms of severe infections. In K18-hACE2 mice CNS had been a major target for SARS-CoV infection which also mimics in SARS-CoV-2 infection as there were attempts to isolate this virus from cerebral microcirculations, cerebrospinal fluids and neuronal tissues (McCray 2007; Bao 2020b). A transgenic mice expressing hACE2 (AC70) showed similar types of infection like clinical signs of illness, weight loss, loss of breath and mortality and infection spread to multiple tissues including the brain (Tseng 2007). hACE2 mice infected with SARS-CoV-2 strain resulted in weight loss but no signs of respiratory illness were reported. Viral antigens could be detected in lungs and intestine but not in other major organs like brain, kidneys and liver (Bao 2020b). The susceptibility, replication and pathogenicity of SARS and SARS-CoV-2 is detailed in Table 4.
Discussions
The genome sequence analysis of SARS-CoV-2 reveals that it shares a sequence identity of 79.6% to SARS-CoV and has 96% identity level of its genome with a novel bat CoV RatG13 indicating its bat origin. The receptor binding spike protein of SARS-CoV-2 had less than 75% sequence homology with previously described all SARS related CoVs but the virus uses ACE2 receptor for cellular entry like other SARS-CoVs and it did not show any affinity for other cellular binding domains like DPP4 and aminopeptidase N. Some SARS-CoV-2 genes shows less than 80% sequence identity with SARS-CoV. There are differences in the spike protein gene between SARS-CoV-2 and SARS-CoV in four out of five key residues of receptor binding motif and three short insertions in N-terminal domain of S-gene (Zhou 2020).
Non-human primates showed no clinical signs of respiratory illness when infected with SARS-CoV strain, however in infection with SARS-CoV-2 strain, initial clinical signs like loss of body weight, hunched posture, loss of appetite are evident. Viral replication in monkeys is also evident in respiratory organs rendering histopathological changes in lungs but extra-pulmonary replication is more evident and pronounced in SARS-CoV-2 infection as compared to SARS-CoV indicating more virulence and pathogenicity. Clinical symptoms and pathological manifestations were observed in common marmosets in SARS-CoV infection but data pertaining to SARS-CoV-2 infection in marmosets is currently not reported.
Higher virulence and fatality of juvenile cats infected with SARS-CoV-2 is reported. Cats are one of the most popular companion animals of humans and are highly vulnerable in SARS-CoV-2 pandemic. There is also a possibility of reverse transmission of virus from infected cats to humans although sufficient data is not available to establish this fact. Ferrets infected with both the viruses show similarities of clinical illness, but infection in ferrets by SARS-CoV-2 is restricted to upper respiratory tract while viral RNA could also be detected in lungs of ferrets in SARS-CoV infection. Hamsters challenged with SARS-CoV strain showed no clinical signs of illness and weight loss but infection with SARS-CoV-2 results in clinical signs of disease. Pigs and chickens are asymptomatic in infections with both SARS-CoV and SARS-CoV-2.
It is also quite notable that the SARS-CoV-2 patients with associated heart disease, diabetes and other systemic disease are more susceptible to fatality. It may be because of multiple disruptions in the system as a result of neurological invasion of SARS-CoV-2 (Baig 2020). Spread of SARS-CoV to multiple organs including brain was demonstrated in K18-hACE2 and hACE2 (AC70) mice from actual site of infection i.e. lungs but interestingly hACE2 transgenic mice infected with SARS-CoV-2 show no presence of viral antigens in brain. SARS-CoV-2 is shown to be highly virulent than SARS-CoV infection resulting in severe signs of respiratory illness and mortality, however, mild symptoms are observed in hACE2 transgenic mice while infected with SARS-CoV-2.
It is quite evident from current knowledge that virulence in animal models with SARS-CoV-2 infection is higher in some of the species as compared to SARS-CoV infection but hACE2 mice are more permissive to infection with SARS-CoV as compared to SARS-CoV-2. The background of strains, age, sex etc. must also be evaluated in species as viral invasion and replication does not solely depend on the entry of virus within the host but host adaptability and immune status also. The testing of new chemical entities, drug repurposing and vaccine evaluations count a good preclinical animal experimentation data, it is high time that research should be directed in searching for reliable and reproducible animal models for emerging coronaviral infections.
References
Baig AM, et al (2020) Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11:995–998. https://doi.org/10.1021/acschemneuro.0c00122
Bao L, et al (2020a) Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. bioRxiv 2020a.03.13.990226. 10.1101/2020a.03.13.990226
Bao L, et al (2020b) The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. https://doi.org/10.1038/s41586-020-2312-y
Chan JF-W, et al (2020) Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa325
Cui J, et al (2019) Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17:181–192. https://doi.org/10.1038/s41579-018-0118-9
Ge X-Y, et al (2013) Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503:535–538. https://doi.org/10.1038/nature12711
Glass WG, et al (2004) Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J Immunol 173:4030–4039. https://doi.org/10.4049/jimmunol.173.6.4030
Greenough TC, et al (2005) Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am J Pathol 167:455–463. https://doi.org/10.1016/S0002-9440(10)62989-6
He B, et al (2014) Identification of diverse alphacoronaviruses and genomic characterization of a novel severe acute respiratory syndrome-like coronavirus from bats in China. J Virol 88:7070–7082. https://doi.org/10.1128/JVI.00631-14
Jeffers SA, et al (2004) CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci U S A 101:15748–15753. https://doi.org/10.1073/pnas.0403812101
Lau SKP, et al (2005) Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A 102:14040–14045. https://doi.org/10.1073/pnas.0506735102
Li W, et al (2004) Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin-converting enzyme 2. J Virol 78:11429–11433. https://doi.org/10.1128/JVI.78.20.11429-11433.2004
Li W, et al (2005a) Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J 24:1634–1643. https://doi.org/10.1038/sj.emboj.7600640
Li W, et al (2005b) Bats are natural reservoirs of SARS-like coronaviruses. Science 310:676–679. https://doi.org/10.1126/science.1118391
McAuliffe J, et al (2004) Replication of SARS coronavirus administered into the respiratory tract of African Green, rhesus and cynomolgus monkeys. Virology 330:8–15. https://doi.org/10.1016/j.virol.2004.09.030
McCray PB, et al (2007) Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol 81:813–821. https://doi.org/10.1128/JVI.02012-06
Menachery VD, et al (2015) SARS-like cluster of circulating bat coronavirus pose threat for human emergence. Nat Med 21:1508–1513. https://doi.org/10.1038/nm.3985
Menachery VD, et al (2016) SARS-like WIV1-CoV poised for human emergence. Proc Natl Acad Sci U S A 113:3048–3053. https://doi.org/10.1073/pnas.1517719113
Roberts A, et al (2005a) Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J Virol 79:5833–5838. https://doi.org/10.1128/JVI.79.9.5833-5838.2005
Roberts A, et al (2005b) Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters. J Virol 79:503–511. https://doi.org/10.1128/JVI.79.1.503-511.2005b
Shi J, et al (2020) Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. https://doi.org/10.1126/science.abb7015
Subbarao K, et al (2004) Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol 78:3572–3577. https://doi.org/10.1128/jvi.78.7.3572-3577.2004
Sumbria D, et al (2019) Factors affecting the tissue damaging consequences of viral infections. Front Microbiol 10:2314. https://doi.org/10.3389/fmicb.2019.02314
Tseng C-TK, et al (2007) Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor. J Virol 81:1162–1173. https://doi.org/10.1128/JVI.01702-06
van den Brand JMA, et al (2008) Pathology of experimental SARS coronavirus infection in cats and ferrets. Vet Pathol 45:551–562. https://doi.org/10.1354/vp.45-4-551
Weingartl HM, et al (2004) Susceptibility of pigs and chickens to SARS coronavirus. Emerg Infect Dis 10:179–184. https://doi.org/10.3201/eid1002.030677
Wentworth DE, et al (2001) Molecular determinants of species specificity in the coronavirus receptor aminopeptidase N (CD13): influence of N-linked glycosylation. J Virol 75:9741–9752. https://doi.org/10.1128/JVI.75.20.9741-9752.2001
Wu D, et al (2005) Civets are equally susceptible to experimental infection by two different severe acute respiratory syndrome coronavirus isolates. J Virol 79:2620–2625. https://doi.org/10.1128/JVI.79.4.2620-2625.2005
Zhou P, et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. https://doi.org/10.1038/s41586-020-2012-7
Acknowledgements
The authors acknowledge the support of CSIR, New Delhi, India and CSIR-CDRI, Lucknow, India. The manuscript bears CSIR-CDRI communication number 10089.
Author information
Authors and Affiliations
Contributions
JS and RG reviewed the literature and drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarkar, J., Guha, R. Infectivity, virulence, pathogenicity, host-pathogen interactions of SARS and SARS-CoV-2 in experimental animals: a systematic review. Vet Res Commun 44, 101–110 (2020). https://doi.org/10.1007/s11259-020-09778-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-020-09778-9